We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
===M22 Agonist=== | ===M22 Agonist=== | ||
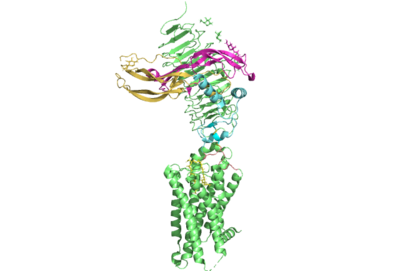
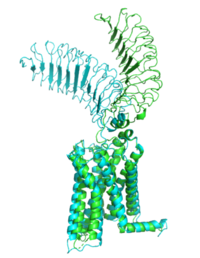
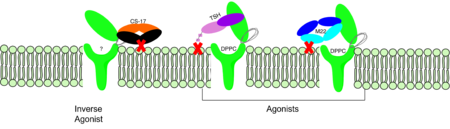
| - | M22 is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, | + | <scene name='95/952708/M22/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR resulting in a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] |
| - | [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] | + | |
| + | ===CS-17 Inverse Agonist=== | ||
| + | CS-17 (GREEN LINK) is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that acts as an inverse agonist for TSHR constitutive activity. <ref name="Chen"> DOI:10.1210/en.2006-1754</ref>. CS-17 interacts with the extracellular domain of the TSHR protein on the convex side of the LRRD. When bound to TSHR, CS-17 suppresses TSHR function by keeping the receptor in the inactive state (Figure 2). Clash with the cell membrane does not allow the inactive form of TSHR to flip to the active conformation. CS-17 plays a unique role with GPCRs. This type of inhibition is not commonly seen in many biological systems and therefore leads to this method of inhibition being a great target for drug design and future research.<ref name="Chen">doi:10.1210/en.2006-1754</ref>. Due to its unique inhibition, CS-17 can be a popular therapy for many thyroid diseases where the thyroid is overactive. | ||
| + | |||
| + | ===TSH Agonist=== | ||
| + | [[Image:NAG.png|200 px|left|thumb|Figure 3]] | ||
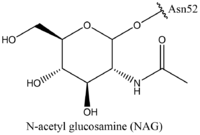
| + | Thyroid-stimulating hormone (TSH) (GREEN LINK) is a hormone that stimulates the thyroid gland to produce proteins that are vital for many metabolic pathways in the body's tissue. TSH activates the TSHR protein by binding to the concave surface of the LRRD and hinge region to keep TSHR in its active state by clashing with the membrane <ref name="Duan"> DOI:10.1038/s41586-022-05173-3</ref>. (Figure 2). This clash is caused by glycosylations of an asparagine 52 residue on the alpha subunit of TSH (GREEN LINK). These modifications to the ASN residue are N-acetyl glucosamine modifications (Figure 3). They stick out from the alpha subunit of TSH to clash with the cell membrane and keep TSH in the active state. | ||
Revision as of 20:54, 2 April 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ 2.0 2.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ . PMID:228484426
- ↑ 5.0 5.1 5.2 5.3 5.4 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 7.0 7.1 doi: https://dx.doi.org/10.1210/en.2006-1754
- ↑ Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3