We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Introduction == | == Introduction == | ||
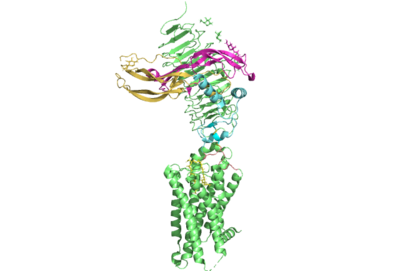
[[Image:All_TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, the P10 peptide in pink, and thyrotropin bound in pink and yellow.]] | [[Image:All_TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, the P10 peptide in pink, and thyrotropin bound in pink and yellow.]] | ||
| - | [https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones | + | [https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones causes many disorders related to [https://www.endocrineweb.com/conditions/thyroid/hyperthyroidism-vs-hypothyroidism hypo- or hyperthyroidism]. Thus, understanding the signaling of synthesis and release of these hormones is essential to developing therapeutic drugs to combat [https://www.hopkinsmedicine.org/health/conditions-and-diseases/disorders-of-the-thyroid specific thyroid hormone disorders]<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The initiation of synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone, commonly referred to as TSH or thyrotropin. The thyrotropin receptor <scene name='95/952709/Initial_scene_with_edited_7utz/2'>(TSHR)</scene> is a [https://www.nature.com/scitable/topicpage/gpcr-14047471/ G-protein coupled receptor] that binds TSH and transduces signal to initiate synthesis and release of thyroid hormones. It is important to note that autoantibodies may also bind to this receptor causing inhibition or activation of its desired function. (Figure 1)<ref name="Duan et al.">PMID:35940204</ref><ref name="Kohn et al.">Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.</ref>. |
=== Grave's Disease === | === Grave's Disease === | ||
| Line 10: | Line 10: | ||
=== Hypothyroidism === | === Hypothyroidism === | ||
| - | Hypothyroidism is the converse of Grave’s Disease as there is not enough TSH produced in the body with this disease. The most common cause of Hypothyroidism is Hashimoto’s disease. Without enough TSH to bind TSHR, the pathway remains inactive and thus metabolic processes are inhibited in this pathway. This results in many symptoms including, but not limited to fatigue, cold sensitivity, weight gain, irregular/heavy menstrual cycle, thinning of hair, and depression. This disease effects women and those older than the age of 60 | + | Hypothyroidism is the converse of Grave’s Disease as there is not enough TSH produced in the body with this disease. The most common cause of Hypothyroidism is Hashimoto’s disease. Without enough TSH to bind TSHR, the pathway remains inactive and thus metabolic processes are inhibited in this pathway. This results in many symptoms including, but not limited to fatigue, cold sensitivity, weight gain, irregular/heavy menstrual cycle, thinning of hair, and depression. This disease effects women and those older than the age of 60, but can also occur in infancy. [https://www.mayoclinic.org/diseases-conditions/hypothyroidism/symptoms-causes/syc-20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages Hypothyroidism] |
== Structure == | == Structure == | ||
===Overview=== | ===Overview=== | ||
| - | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in | + | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">PMID:228484426</ref>. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective <scene name='95/952709/G_protein/2'>G-protein</scene> when in active and inactive states. |
| + | |||
=== Leucine Rich Region === | === Leucine Rich Region === | ||
| - | The Leucine Rich region is part of the <scene name='95/952708/Tshr_chainr_ecd/1'> | + | The Leucine Rich region (LRRD) is part of the <scene name='95/952708/Tshr_chainr_ecd/1'>ECD</scene> of TSHR. The highlighted region contains <scene name='95/952707/Lrr/3'>10-11 Leucine Repeats</scene> within the structure. The specific residues from TSHR interacting with TSH are <scene name='95/952707/Lrr/2'>K209 and K58</scene> <ref name="Duan et al.">PMID: 35940204</ref>. These interact with <scene name='95/952709/Interactions_with_thyrotropin/1'> E98 and N91</scene> in the seatbelt region of TSH forming a salt bridge and initiating the conformational change in the receptor <ref name="Faust">PMID: 35940205</ref>. This interaction is specific to TSH and TSHR. When other agonists or antagonists bind to the receptor, the change in conformation is a result of different residues interacting. The Leucine residues likely play a role in how the ECD folds and which residues are located on the exterior protein. As Leucine is hydrophobic, it would be forced into the interior of the protein during folding exposing other residues that are more hydrophilic and likely to interact externally. |
===Hinge Region and P10 Peptide=== | ===Hinge Region and P10 Peptide=== | ||
| - | Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the ECD to the 7TMD. | + | Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the ECD to the 7TMD. This region has also been found to have impact on the binding potency of TSH as well as intracellular cyclic adenosine monophosphate (cAMP) levels, which are partially mediated by the activation of the GPCR. Several features of this region have been found to be crucial to the activation of the TSHR by TSH.<ref name="Mizutori et al.">Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407</ref>. The most important feature of the hinge region is the interaction of the <scene name='95/952709/Hinge_helix_rotation/1'>hinge helix</scene> with the <scene name='95/952709/P10_peptide_region/2'>p10 peptide</scene> through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1)<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. These disulfides are critical because they link the ECD (where thyrotropin binds) to the TMD, whose conformational changes directly activate the receptor's complementary G-protein. The movement of the ECD, which is caused by TSH binding, will cause rotation of the hinge helix and movement of the p10 peptide. This leads to movement of the transmembrane helices which cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing Y385 and D386. Y385 is buried into a hydrophobic region of TSH while D386 forms a salt bride with R386 of the hormone. Together, <scene name='95/952709/Binding_interactions_hinge/1'>these interactions</scene> facilitate the stable binding of TSH to the TSHR <ref name="Duan et al.">PMID:35940204</ref>. Additionally, it is important to acknowledge the hinge region itself is not required for the activation of the receptor. In many of the aforementioned misregulations of thyroid hormone release, auto-antibodies are responsible. These auto-antibodies bind to the receptor through alternative interactions which cause conformational changes to the 7TMD without any need for a conformational change or extensive interactions with the hinge region<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. |
===7 Transmembrane Helices=== | ===7 Transmembrane Helices=== | ||
| - | The thyrotropin receptor is anchored to the membrane through seven transmembrane helices which is characteristic of GPCRs. Conformational changes in this region of the receptor are responsible for the activation of associated G protein<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. In thyrotropin binding, changes to this region are mediated by movements in the | + | The thyrotropin receptor is anchored to the membrane through seven transmembrane helices which is characteristic of GPCRs. Conformational changes in this region of the receptor are responsible for the activation of associated G protein<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. In thyrotropin binding, changes to this region are mediated by movements in the p10 peptide. When the hinge helix rotates, it causes p10 peptide displacement that allows the <scene name='95/952709/Helix_7_of_7tmd/2'>seventh helix</scene> of the transmembrane domain to migrate towards the center of the 7TMD to increase Van der Waals contacts. In addition, K660 of TM7 forms an <scene name='95/952709/Helix_7_and__p10_interaction_2/1'>ionic interaction</scene> with glutamate 409 of the p10 region. This interaction assists in the stabilization of the active state of 7TMD. In addition, the movement of the hinge helix has been found to bee associated with movement of a tyrosine residue relative to and isoleucine residue on the neighboring extracellular loop 1 (ECL1) helix. The identity of these residues has been found to be an important predictor in the activation of the thyrotropin receptor. For instance, structurally guided mutagenic studies have shown that the mutation of the isoleucine to a more sizeable phenylalanine decreases TSH signaling potency<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Vlaeminck-Guillem et al.">Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816</ref>.In addition to these conformational changes, the sixth transmembrane helix of TSHR is also moved outward from the center of the 7TMD to facilitate alpha helix 5 of the alpha subunit of the G protein, which is the domain that becomes activated<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Goricanec et al.">Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113 </ref>. |
=== Active and Inactive Form === | === Active and Inactive Form === | ||
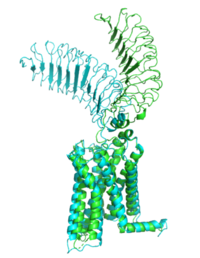
[[Image:Morph_pics2.png|200 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | [[Image:Morph_pics2.png|200 px|right|thumb|Figure 1: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.]] | ||
| - | The TSHR protein exists in two states | + | The TSHR protein exists in two states: active and inactive (Figure 1). The <scene name='95/952708/Tshr_chainr_ecd/1'>ECD</scene> protrudes from the cell membrane into the space outside the cell. The <scene name='95/952708/Tshr_chainr_tm/1'>transmembrane domain</scene> contains 7 alpha helices that reside within the cell membrane. The <scene name='95/952708/Tshr_chainr/4'>TSHR active form</scene> exists when bound to the TSH (GREEN LINK). One proposed mechanism for the transition from the active to inactive describes that in a natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.<ref name="Faust" /> Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. <ref name="Faust" /> |
== TSHR Agonists and Antagonists == | == TSHR Agonists and Antagonists == | ||
| - | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/Receptor_antagonist antagonists] block or inhibit biological processes. Different types of agonists/antagonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead | + | Chemical [https://en.wikipedia.org/wiki/Agonist agonists] are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical [https://en.wikipedia.org/wiki/Receptor_antagonist antagonists] block or inhibit biological processes. Different types of agonists/antagonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead researchers to uncover the mechanism of binding for the receptor. |
===M22 Agonist=== | ===M22 Agonist=== | ||
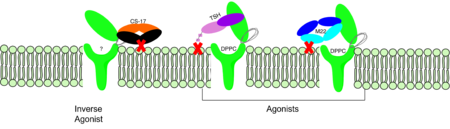
| - | <scene name='95/952708/M22/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR | + | <scene name='95/952708/M22/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that was isolated from a patient with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, TSHR autoantibodies like M22 mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 2). This autoantibody mimics TSH action and binding to TSHR, indicating M22 is a potent activator for TSHR. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, there is a key difference in binding between the two that can reveal the function of the hinge region (GREEN LINK). M22 does not make interactions with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 2: Agonist and antagonist drugs for activating or inactivating the TSHR protein.]] |
===CS-17 Inverse Agonist=== | ===CS-17 Inverse Agonist=== | ||
| - | CS-17 (GREEN LINK) is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that acts as an inverse agonist for TSHR constitutive activity. <ref name="Chen"> DOI:10.1210/en.2006-1754</ref>. CS-17 interacts with the | + | CS-17 (GREEN LINK) is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that acts as an inverse agonist for TSHR constitutive activity. <ref name="Chen"> DOI:10.1210/en.2006-1754</ref>. CS-17 interacts with the ECD of the TSHR protein on the convex side of the LRRD. When bound to TSHR, CS-17 suppresses TSHR function by keeping the receptor in the inactive state (Figure 2). Clash with the cell membrane does not allow the inactive form of TSHR to flip to the active conformation. CS-17 plays a unique role with GPCRs. This type of inhibition is not commonly seen in many biological systems and therefore leads to this method of inhibition being a great target for drug design and future research.<ref name="Chen">doi:10.1210/en.2006-1754</ref>. Due to its unique inhibition, CS-17 can be a popular therapy for many thyroid diseases where the thyroid is overactive. |
===TSH Agonist=== | ===TSH Agonist=== | ||
[[Image:NAG.png|200 px|left|thumb|Figure 3]] | [[Image:NAG.png|200 px|left|thumb|Figure 3]] | ||
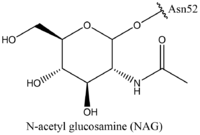
| - | + | TSH (GREEN LINK), as previously mentioned, is a hormone that stimulates the thyroid gland to produce proteins that are vital for many metabolic pathways in the body's tissue. TSH activates the TSHR protein by binding to the concave surface of the LRRD and hinge region to keep TSHR in its active state by clashing with the membrane <ref name="Duan"> DOI:10.1038/s41586-022-05173-3</ref>. (Figure 2). This clash is caused by glycosylations of an N52 residue on the alpha subunit of TSH (GREEN LINK). These modifications to the N residue are N-acetyl glucosamine modifications (Figure 3). They stick out from the alpha subunit of TSH to clash with the cell membrane and keep TSH in the active state. | |
Revision as of 21:24, 2 April 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ 2.0 2.1 2.2 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 4.0 4.1 . PMID:228484426
- ↑ 5.0 5.1 5.2 5.3 5.4 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 7.0 7.1 7.2 7.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 11.0 11.1 doi: https://dx.doi.org/10.1210/en.2006-1754
- ↑ Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3