Thyroid Stimulating Hormone Receptor (TSHR) Structure and Function
Introduction
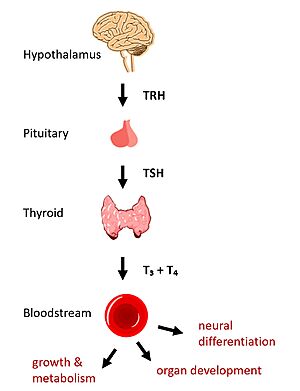
Figure 1: TSHR binds TSH in the HPT signaling axis pathway, which regulates metabolism and growth.
In humans, the hypothalamic-pituitary-thyroid (HPT) signaling axis regulates functions including metabolism, growth, organ development, and neural differentiation [1]. In this pathway, the thyroid stimulating hormone receptor (TSHR) activates transcription of thyroid hormones in response to ligand binding by thyroid stimulating hormone (TSH). After a brief introduction to the biological significance of TSHR, this page explores the structure of TSHR and its significance to TSH binding and receptor activation.
Biological Significance of TSHR
The HPT signaling axis involves the brain, thyroid gland, and bloodstream circulation. In the first step of the pathway, thyrotropin releasing hormone (TRH) is secreted by the hypothalamus, which in turn stimulates the anterior pituitary gland to produce TSH [1]. TSH binds to TSHR on the surface of thyroid cells and triggers the production of thyroid hormones thyroxine (T3) and triiodothyronine (T4) through G-protein coupled receptor (GPCR) signaling [2]. T3 and T4 circulate in the bloodstream and enter cells via thyroid hormone transporters to regulate metabolic functions. Additionally, T3 and T4 act in a negative feedback loop to inhibit further TSH production [1].
Dysregulation of TSHR can lead to disease. In Grave's disease, antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism [2]. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated [1].
Structural Overview of TSHR
Describe the domains here.
TSHR Activation
Hinge Motion
Central to the biological function of TSHR is its hinge motion which allows for transition between active and inactive states. Deformation of the hinge region accommodates up-and-down rotation of the extracellular domain as a rigid body about an imaginary 55 degree axis. When the extracellular domain is upright, the receptor actively signals for thyroid hormone production. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. Notably, transition between the two states occurs spontaneously; favoring of the active or inactive conformation is influenced by hinge interactions and ligand binding [3].
At the following link is an animation of TSHR transition between the two states: Image:TSHR MorphBetterAngle.mp4. The following trends can be observed in the video or viewed through an in the molecule viewer:
- Slinky-like deformation of the hinge region shifts the region 5 Angstroms over the course of the movement [3].
- Stretching of the hinge pulls on the linked helices in the transmembrane domain, shifting about 4 Angstroms inward [3].
Stabilizing Interactions in the Hinge
To understand stabilizing interactions which accommodate the hinge motion, the hinge region can be subdivided into the , which lies at the intersection of the extracellular and transmembrane domains; , which sticks up and serves as a binding platform for the TSH ligand; the region, which connects helix 1 with the p10 region; and the region, a conserved 10-amino acid sequence which connects to transmembrane helix 7 and undergoes most of the deformation [3] [4].
Two key disulfide bridges within the hinge region help to maintain its structure and orientation [4].
- The connects the hinge helix with the linker region
- The connects the hinge helix with the p10 region.
The upright, active conformation of the hinge is stabilized by its respective interactions wit the EC and TM domains [3].
- A occurs between Y279 in the hinge helix and I486 in EC loop region 1, which protrudes from the TM helices.
- An occurs between K660 in TM helix 7 and E409 in the p10 region.
If the stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine strengthens the hydrophobic interaction too much leading to overactivation. Contrastingly, TSHR underactivation results from disrupting the ionic interaction with an E409A mutation, which is associated with diminished receptor activation and TSH potency [3].
Ligand Binding
Ligand-induced Conformation Change
Describe how TSH binds in the upright conformation here.
Ligand Recognition
Describe seatbelt and hinge important to ligand recognition here.
This shows the TSHR hinge movement.
.
References
- ↑ 1.0 1.1 1.2 1.3 Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-3043. DOI: 10.1172/JCI60047
- ↑ 2.0 2.1 Chu YD, Yeh CT. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells. 2020;9(7):1730. DOI:10.3390/cells9071730
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 4.0 4.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3

