We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Glycogen Phosphorylase
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
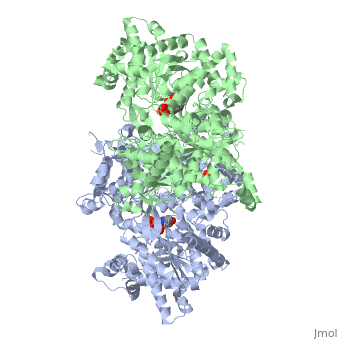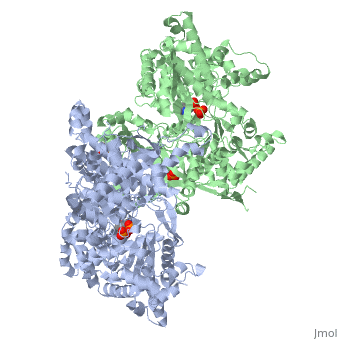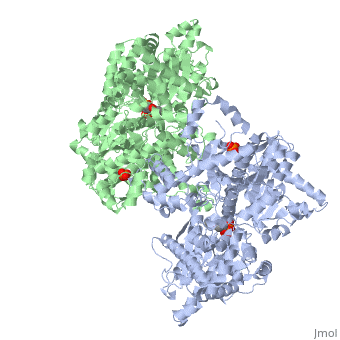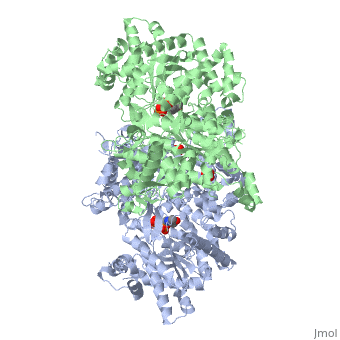
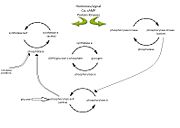
Glycogen phosphorylase is a dimer consisting of two identical subunits and has an essential cofactor, <scene name='Sandbox_153/Plp/1'>pryridoxal phosphate (PLP)</scene><ref name="PLP"/>. Glycogen phosphorylase can be found in two different states, glycogen phosphorylase a (GP''a'') and glycogen phosphorylase b (GP''b'')<ref name="gp"/>The difference in the structures is due to phosphorylation of the <scene name='Sandbox_153/Ser14/1'>Ser-14</scene> residue which results in the active form (GP''a''). Protein phosphatases dephosphorylate the GP''a'' to the inactive form, also known as GP''b''. Both forms of glycogen phosphorylase can also be found in T and R states where T is the inactive state because it appears to have a low affinity for substrate and R is the active state where it appears to have a greater affinity for substrate<ref name="gp2">PMID: 1900534</ref>. | Glycogen phosphorylase is a dimer consisting of two identical subunits and has an essential cofactor, <scene name='Sandbox_153/Plp/1'>pryridoxal phosphate (PLP)</scene><ref name="PLP"/>. Glycogen phosphorylase can be found in two different states, glycogen phosphorylase a (GP''a'') and glycogen phosphorylase b (GP''b'')<ref name="gp"/>The difference in the structures is due to phosphorylation of the <scene name='Sandbox_153/Ser14/1'>Ser-14</scene> residue which results in the active form (GP''a''). Protein phosphatases dephosphorylate the GP''a'' to the inactive form, also known as GP''b''. Both forms of glycogen phosphorylase can also be found in T and R states where T is the inactive state because it appears to have a low affinity for substrate and R is the active state where it appears to have a greater affinity for substrate<ref name="gp2">PMID: 1900534</ref>. | ||
| - | The secondary structures of T and R states of GP''a'' and ''b'' are similar with an <scene name='Sandbox_153/Nterminaldomain/2'>N-terminal domain </scene> and a <scene name='Sandbox_153/Cterminaldomain/1'>C-terminal domain </scene>.Each domain also contains subdomains which undergo conformational changes on the interconversion of T and R states <ref name="gp2"/>. C terminal domain has the cofactor PLP and part of the active site, it is made up of five α helices and 6 β strands<ref name="structureandfunction">Fletterick RJ, Sprang SR. Glycogen phosphorylase Structures and function. Accounts of Chemical Research. 1982 Nov; 15(11):361-369.</ref>. The N-terminal domain consisting of fifteen α helices and nine β strands, is considered to be more complex and is divided in the middle of its β sheet core into subdomains. The first domain binds the effector molecule of AMP and also has a recognition site of the introconverting phosphorylase kinase and phosphatase<ref name="gp2"/><ref name="structureandfunction"/>. The second domain has the polysaccharide binding domain where phosphorylase is able to attach to the glycogen substrate <ref name="structureandfunction"/>. The R states of GP''a'' and GP''b'' are almost identical; the difference lays in the modification of the Ser-14 residue where GP''a'' has a covalently linked phosphate group whereas GP''b'' has a non-covalently linked sulfide group . GP''a'' is activated by phosphorylation of the serine residue whereas GP''b'' can be activated by the binding of AMP to the <scene name='Sandbox_153/Activesites/3'>allosteric sites </scene> that are present within the molecule<ref name="gp2"/>. GP''a'' does not require the binding of AMP but attachment enhances the activity of the enzyme upwards to 25% <ref name="allosteric"> Johnson LH. Glycogen Phosphorylase: Control by phosphorylation and allosteric effectors. The FASEB Journal. 1992 March;6:2274-2282.</ref>. | + | The secondary structures of T and R states of GP''a'' and ''b'' are similar with an <scene name='Sandbox_153/Nterminaldomain/2'>N-terminal domain </scene> and a <scene name='Sandbox_153/Cterminaldomain/1'>C-terminal domain </scene>.Each domain also contains subdomains which undergo conformational changes on the interconversion of T and R states <ref name="gp2"/>. C terminal domain has the cofactor PLP and part of the active site, it is made up of five α helices and 6 β strands<ref name="structureandfunction">Fletterick RJ, Sprang SR. Glycogen phosphorylase Structures and function. Accounts of Chemical Research. 1982 Nov; 15(11):361-369.</ref>. The N-terminal domain consisting of fifteen α helices and nine β strands, is considered to be more complex and is divided in the middle of its β sheet core into subdomains. |
| + | The first domain binds the effector molecule of AMP and also has a recognition site of the introconverting phosphorylase kinase and phosphatase<ref name="gp2"/><ref name="structureandfunction"/>. The second domain has the polysaccharide binding domain where phosphorylase is able to attach to the glycogen substrate <ref name="structureandfunction"/>. The R states of GP''a'' and GP''b'' are almost identical; the difference lays in the modification of the <scene name='38/382926/Ser14_interactions/1'>Ser-14</scene> residue where GP''a'' has a covalently linked phosphate group whereas GP''b'' has a non-covalently linked sulfide group . GP''a'' is activated by phosphorylation of the serine residue whereas GP''b'' can be activated by the binding of AMP to the <scene name='Sandbox_153/Activesites/3'>allosteric sites </scene> that are present within the molecule<ref name="gp2"/>. GP''a'' does not require the binding of AMP but attachment enhances the activity of the enzyme upwards to 25% <ref name="allosteric"> Johnson LH. Glycogen Phosphorylase: Control by phosphorylation and allosteric effectors. The FASEB Journal. 1992 March;6:2274-2282.</ref>. | ||
Glycogen phosphorylase is different from other enzymes that require the cofactor PLP because instead of utilizing the pyrimidine ring, phosphorylase uses the phosphate group <ref name="allosteric"/>. The 4'aldehyde of PLP binds to the ε-amino group of lysine 680 and the 5'-phosphate of PLP has been found to be the group participating in the catalysis of glycogen phosphorylase <ref name="PLP"/>. The binding sites in glycogen phosphorylase include: a catalytic, inhibiting, AMP, glycogen and new allosteric site <ref name="gp"/>. The glycogen binding site is located more than 30Å from the catalytic and allosteric sites. The residues that make up the site are <scene name='Sandbox_153/Glycogen_binding_site/1'>Arg426, Glu433, Gly434, and Ala435</scene><ref name="allosteric"/>. The <scene name='Sandbox_153/Inhibitorbindingsite/1'>inhibitor site</scene>binds purine analogs or fused-ring molecules such as adenosine, caffeine, FMN, NADH and AMP when there are increased concentrations available<ref name="gp2"/>. The heterocyclic rings of the compounds bind to the inhibiting site, stablilizing it and blocking access to the catalytic center <ref name="allosteric"/>. The <scene name='Sandbox_153/Catalyticsite/1'>catalytic site</scene> can be accessed once the Ser-14 residue has been phosphorylated and conformational changes in glycogen phosphorylation have been observed<ref name="structureandfunction"/><ref name="allosteric"/>. The structure and function of glycogen phosphorylase is complex, though the function of the enzyme is due to the structure. | Glycogen phosphorylase is different from other enzymes that require the cofactor PLP because instead of utilizing the pyrimidine ring, phosphorylase uses the phosphate group <ref name="allosteric"/>. The 4'aldehyde of PLP binds to the ε-amino group of lysine 680 and the 5'-phosphate of PLP has been found to be the group participating in the catalysis of glycogen phosphorylase <ref name="PLP"/>. The binding sites in glycogen phosphorylase include: a catalytic, inhibiting, AMP, glycogen and new allosteric site <ref name="gp"/>. The glycogen binding site is located more than 30Å from the catalytic and allosteric sites. The residues that make up the site are <scene name='Sandbox_153/Glycogen_binding_site/1'>Arg426, Glu433, Gly434, and Ala435</scene><ref name="allosteric"/>. The <scene name='Sandbox_153/Inhibitorbindingsite/1'>inhibitor site</scene>binds purine analogs or fused-ring molecules such as adenosine, caffeine, FMN, NADH and AMP when there are increased concentrations available<ref name="gp2"/>. The heterocyclic rings of the compounds bind to the inhibiting site, stablilizing it and blocking access to the catalytic center <ref name="allosteric"/>. The <scene name='Sandbox_153/Catalyticsite/1'>catalytic site</scene> can be accessed once the Ser-14 residue has been phosphorylated and conformational changes in glycogen phosphorylation have been observed<ref name="structureandfunction"/><ref name="allosteric"/>. The structure and function of glycogen phosphorylase is complex, though the function of the enzyme is due to the structure. | ||
Revision as of 10:19, 3 April 2023
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
- ↑ 1.0 1.1 1.2 Kristiansen M, Andersen B, Iversen LF, Westergaard N. Identification, synthesis, and characterization of new glycogen phosphorylase inhibitors binding to the allosteric AMP site. J Med Chem. 2004 Jul 1;47(14):3537-45. PMID:15214781 doi:10.1021/jm031121n
- ↑ 2.0 2.1 Roach PJ. Glycogen and its metabolism. Curr Mol Med. 2002 Mar;2(2):101-20. PMID:11949930
- ↑ 3.0 3.1 3.2 3.3 Palm D, Klein HW, Schinzel R, Buehner M, Helmreich EJM. The role of pyridoxal 5’-phosphate in glycogen phosphorylase catalysis. Biochemistry. 1990 Feb 6; 29(5):1099-1107.
- ↑ 4.0 4.1 4.2 4.3 4.4 Barford D, Hu SH, Johnson LN. Structural mechanism for glycogen phosphorylase control by phosphorylation and AMP. J Mol Biol. 1991 Mar 5;218(1):233-60. PMID:1900534
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Fletterick RJ, Sprang SR. Glycogen phosphorylase Structures and function. Accounts of Chemical Research. 1982 Nov; 15(11):361-369.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Johnson LH. Glycogen Phosphorylase: Control by phosphorylation and allosteric effectors. The FASEB Journal. 1992 March;6:2274-2282.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Amy Chahal, Ann Taylor, Alexander Berchansky, Joel L. Sussman, Riley Hicks, Andrea Gorrell, David Canner