We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1769
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
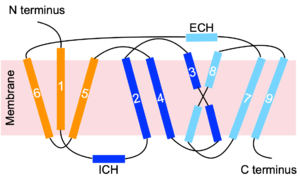
NTCP is a transmembrane protein found in hepatocyte cells. It consists of nine <scene name='95/952697/Ntcp_open-pore_state/22'>transmembrane alpha helices</scene>, with the N-terminus located on the extracellular side of the plasma membrane and the C-terminus located on the intracellular side (Figure 3). Transmembrane helices are connected by short loops as well as extracellular and intracellular alpha helices that lie nearly parallel to the membrane.<ref name="Asami" /> Structures were determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryogenic electron microscopy (Cryo-EM)] of NTCP in complex with antibodies or nanobodies. <ref name="Goutam" /> <ref name="Asami" /> <Ref name="Park"> Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. [https://dx.doi.org/10.1038/s41586-022-04857-0 DOI: 10.1038/s41586-022-04857-0]. </Ref> <ref name="Liu" /> | NTCP is a transmembrane protein found in hepatocyte cells. It consists of nine <scene name='95/952697/Ntcp_open-pore_state/22'>transmembrane alpha helices</scene>, with the N-terminus located on the extracellular side of the plasma membrane and the C-terminus located on the intracellular side (Figure 3). Transmembrane helices are connected by short loops as well as extracellular and intracellular alpha helices that lie nearly parallel to the membrane.<ref name="Asami" /> Structures were determined by [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy cryogenic electron microscopy (Cryo-EM)] of NTCP in complex with antibodies or nanobodies. <ref name="Goutam" /> <ref name="Asami" /> <Ref name="Park"> Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. [https://dx.doi.org/10.1038/s41586-022-04857-0 DOI: 10.1038/s41586-022-04857-0]. </Ref> <ref name="Liu" /> | ||
| - | [[Image:Topology_picture.png|300 px|thumb|Figure 3. NTCP | + | [[Image:Topology_picture.png|300 px|thumb|Figure 3. NTCP topology.]] |
=== Domains === | === Domains === | ||
| Line 26: | Line 26: | ||
=== Mechanism of Bile Salt Uptake === | === Mechanism of Bile Salt Uptake === | ||
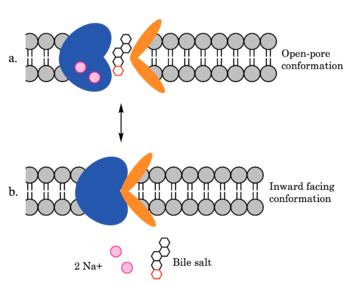
| - | [[Image:ntcpmechanismoverall.png|350 px|right|thumb| Figure 4. Mechanism of | + | [[Image:ntcpmechanismoverall.png|350 px|right|thumb| Figure 4. Mechanism of bile salt uptake by NTCP.]] |
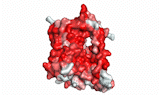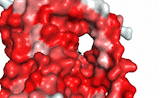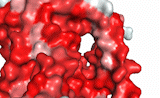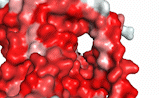
NTCP utilizes secondary active transport to uptake bile salts from blood into the cytoplasm of liver cells (Figure 4). NTCP is crucial for bile salt homeostasis. Specific substrate binding pockets for bile salts have not been identified on NTCP, so the exact mechanism of uptake is unknown. However, it is known that bile salts recognize and bind to the <scene name='95/952697/Ntcp_open-pore_state_surface/4'>open-pore state</scene> (Figure 4a), characterized by an exposed region on the extracellular side. After binding, bile salts pass through the amphipathic pore [[Image:Hydro_NEW_AdobeExpress_(1).gif|200 px|right|thumb|Figure 5. Amphipathic pore of NTCP colored by hydrophobicity. Hydrophobic residues are colored red while hydrophilic residues are colored white.]](Figure 5), and NTCP transitions into the <scene name='95/952697/Ntcp_inward_facing_state/3'>inward facing state</scene> (Figure 4b). In this conformation, the pore closes off relative to the extracellular side and opens to the cytoplasmic side. Transition to the inward facing state allows release of bile salts and sodium ions. It is not yet known how this transition exactly proceeds.<ref name="Asami" /> | NTCP utilizes secondary active transport to uptake bile salts from blood into the cytoplasm of liver cells (Figure 4). NTCP is crucial for bile salt homeostasis. Specific substrate binding pockets for bile salts have not been identified on NTCP, so the exact mechanism of uptake is unknown. However, it is known that bile salts recognize and bind to the <scene name='95/952697/Ntcp_open-pore_state_surface/4'>open-pore state</scene> (Figure 4a), characterized by an exposed region on the extracellular side. After binding, bile salts pass through the amphipathic pore [[Image:Hydro_NEW_AdobeExpress_(1).gif|200 px|right|thumb|Figure 5. Amphipathic pore of NTCP colored by hydrophobicity. Hydrophobic residues are colored red while hydrophilic residues are colored white.]](Figure 5), and NTCP transitions into the <scene name='95/952697/Ntcp_inward_facing_state/3'>inward facing state</scene> (Figure 4b). In this conformation, the pore closes off relative to the extracellular side and opens to the cytoplasmic side. Transition to the inward facing state allows release of bile salts and sodium ions. It is not yet known how this transition exactly proceeds.<ref name="Asami" /> | ||
=== Mechanism of HBV/HDV Infection === | === Mechanism of HBV/HDV Infection === | ||
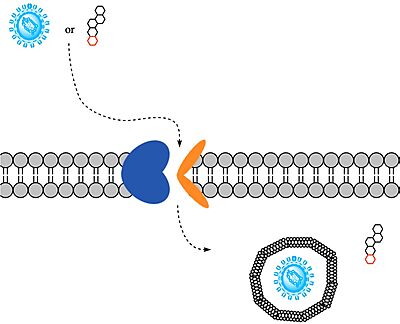
HBV and HDV viruses are transported through NTCP via secondary active transport. After binding to NTCP in the <scene name='95/952697/Ntcp_open-pore_state/26'>open-pore state</scene>, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of the virus occurs. Once inside the cell, the viral genetic information is released. | HBV and HDV viruses are transported through NTCP via secondary active transport. After binding to NTCP in the <scene name='95/952697/Ntcp_open-pore_state/26'>open-pore state</scene>, the viruses remain bound until low bile salt levels in the blood shift equilibria enough that [https://en.wikipedia.org/wiki/Endocytosis endocytosis] of the virus occurs. Once inside the cell, the viral genetic information is released. | ||
| - | The exact mechanism of how HBV and HDV bind to NTCP is not certain, although Park et. al has identified <scene name='95/952696/Residues_84-87_and_157-165_new/1'>two critical sites</scene> on NTCP: residues <scene name='95/952696/Residues_84-87_new/1'>84-87</scene> and <scene name='95/952696/Residues_157-165_new/1'>157-165</scene>. An additional [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] was discovered in East Asia involving <scene name='95/952696/Residue_267/3'> | + | The exact mechanism of how HBV and HDV bind to NTCP is not certain, although Park et. al has identified <scene name='95/952696/Residues_84-87_and_157-165_new/1'>two critical sites</scene> on NTCP: residues <scene name='95/952696/Residues_84-87_new/1'>84-87</scene> and <scene name='95/952696/Residues_157-165_new/1'>157-165</scene>. An additional [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism] was discovered in East Asia involving <scene name='95/952696/Residue_267/3'>residue 267</scene> being mutated from serine to phenylalanine. This mutation prevented HBV/HDV infection. Another mutation, replacing <scene name='95/952696/Leucine_residues/2'>L27, L31, and L35</scene> with tryptophan presumably blocks the preS1 binding site preventing proper HBV/HDV infection. It has also been shown that [https://en.wikipedia.org/wiki/Myristoylation myristoylation] of the HBV/HDV capsid is vital for recognition by NTCP, as well as residues 8-17 on HBV/HDV (sequence: NPLGFFPDHQ)<ref name="Park"/>. There are two proposed mechanisms for how HBV/HDV binds to NTCP. The first proposes binding of the myristoyl group to the host cell membrane, while residues 8-17 interact with NTCP residues 157-165. The second proposes binding of the myristoyl group with residues 157-165 in the pore.<Ref name="Zhang"> Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. [https://dx.doi.org/10.1016/j.biomaterials.2014.04.037 DOI: 10.1016/j.biomaterials.2014.04.037]. </Ref> |
== Medical Relevance == | == Medical Relevance == | ||
Revision as of 17:47, 3 April 2023
Sodium-taurocholate Co-transporting Polypeptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Goutam K, Ielasi FS, Pardon E, Steyaert J, Reyes N. Structural basis of sodium-dependent bile salt uptake into the liver. Nature. 2022 Jun;606(7916):1015-1020. DOI: 10.1038/s41586-022-04723-z.
- ↑ 2.0 2.1 2.2 2.3 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 3.0 3.1 Park JH, Iwamoto M, Yun JH, Uchikubo-Kamo T, Son D, Jin Z, Yoshida H, Ohki M, Ishimoto N, Mizutani K, Oshima M, Muramatsu M, Wakita T, Shirouzu M, Liu K, Uemura T, Nomura N, Iwata S, Watashi K, Tame JRH, Nishizawa T, Lee W, Park SY. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature. 2022 Jun;606(7916):1027-1031. DOI: 10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 4.2 Liu H, Irobalieva RN, Bang-Sørensen R, Nosol K, Mukherjee S, Agrawal P, Stieger B, Kossiakoff AA, Locher KP. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res. 2022 Aug;32(8):773-776. DOI: 10.1038/s41422-022-00680-4.
- ↑ Qi X, Li W. Unlocking the secrets to human NTCP structure. Innovation (Camb). 2022 Aug 1;3(5):100294. DOI: 10.1016/j.xinn.2022.100294.
- ↑ 6.0 6.1 Zhang X, Zhang Q, Peng Q, Zhou J, Liao L, Sun X, Zhang L, Gong T. Hepatitis B virus preS1-derived lipopeptide functionalized liposomes for targeting of hepatic cells. Biomaterials. 2014 Jul;35(23):6130-41. DOI: 10.1016/j.biomaterials.2014.04.037.
- ↑ Patton JS, Carey MC. Watching fat digestion. Science. 1979 Apr 13;204(4389):145-8. DOI: 10.1126/science.432636.
- ↑ Donkers JM, Kooijman S, Slijepcevic D, Kunst RF, Roscam Abbing RL, Haazen L, de Waart DR, Levels JH, Schoonjans K, Rensen PC, Oude Elferink RP, van de Graaf SF. NTCP deficiency in mice protects against obesity and hepatosteatosis. JCI Insight. 2019 Jun 25;5(14):e127197. DOI: 10.1172/jci.insight.127197.
Student Contributors
- Ben Minor
- Maggie Samm
- Zac Stanley