Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 32: | Line 32: | ||
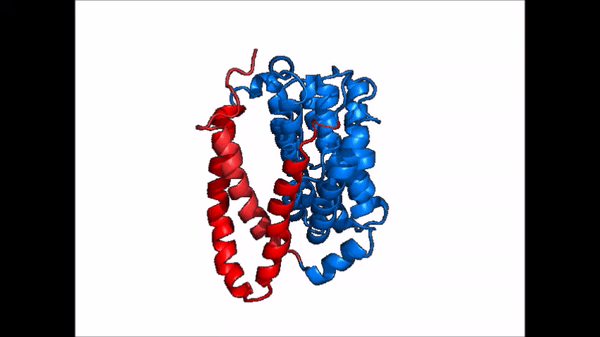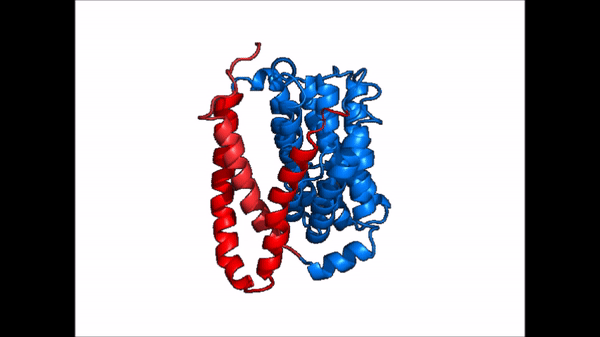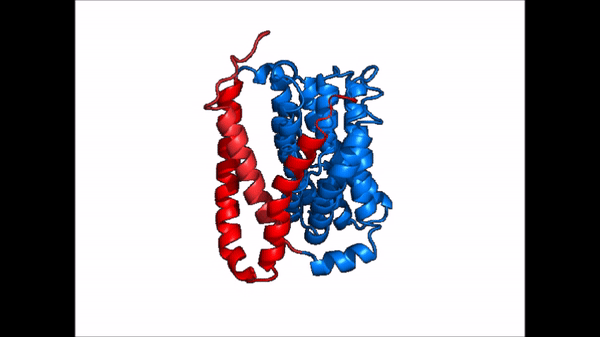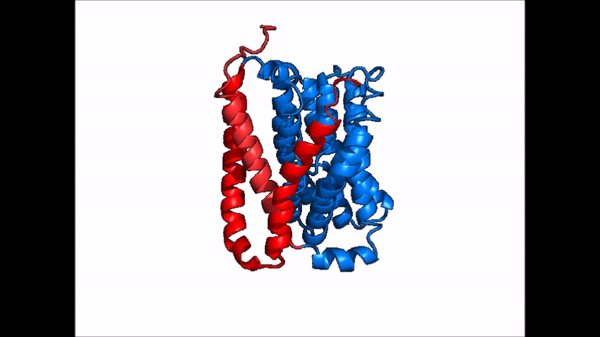
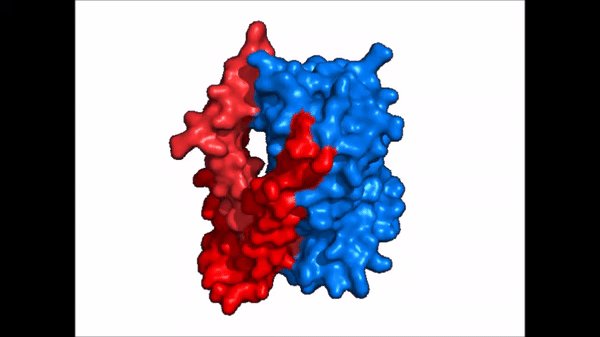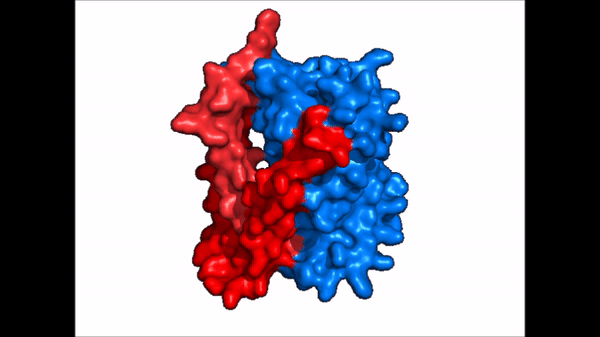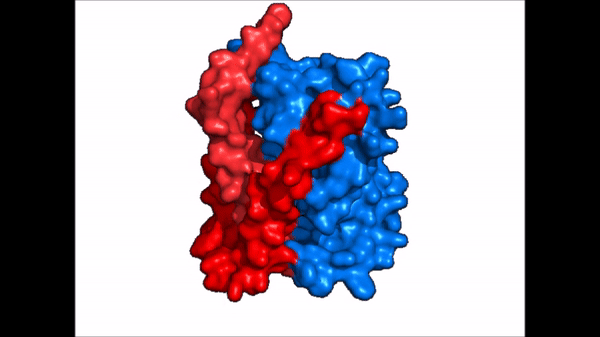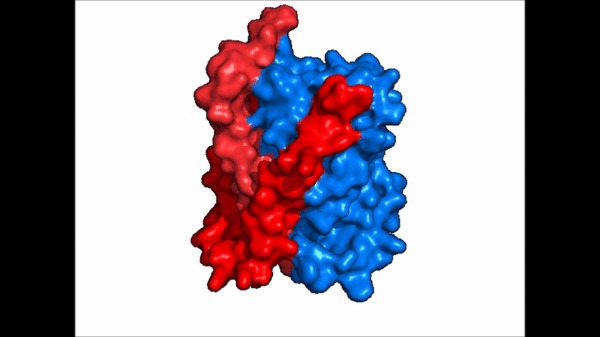
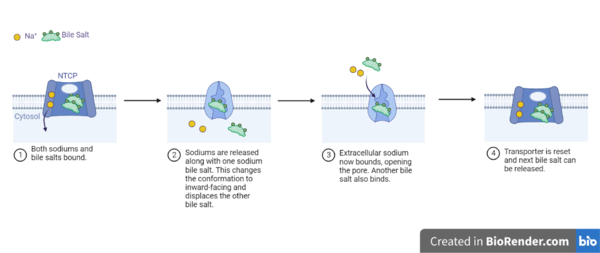
[[Image:NTCP_mech.png|left|600 px|thumb| '''Figure 5: Diagram of Proposed Bile Salt Transport Process''']] | [[Image:NTCP_mech.png|left|600 px|thumb| '''Figure 5: Diagram of Proposed Bile Salt Transport Process''']] | ||
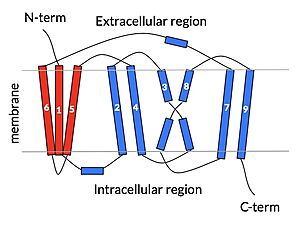
| - | A proposed pathway for NTCP bile salt transport suggests that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref> | + | A proposed pathway for NTCP bile salt transport suggests that both sodium ions are translocated with the transport of one bile salt.<Ref name = "Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </Ref> Initally all <scene name='95/952721/Mech_step_1/1'>ligands and sodium ions are bound</scene> then both sodium ions are released along with the inner bile salt into the cytoplasm (Fig. 5). The <scene name='95/952721/Mech_step_2/2'>outermost bile salt remains bound</scene> however in the pore, likely helping to prevent leakage. <Ref name = "Liu"/> The <scene name='95/952721/Mech_step_3/2'> outer bile salt is displaced </scene> into the inner bile salt placement by the movement of sodium ions that facilitates the conformational change to the inward-facing, pore inaccessible conformation (Fig. 5). <Ref name = "Liu"/> It utilizes an [https://www.sciencedirect.com/science/article/pii/S0092867417302891 elevator-alternating mechanism] where one domain <font color='#6060ff'><b>(core)</b></font> does most of the translocation, and the other domain <font color='red'><b>(panel)</b></font> remains stationary. <Ref name = "Asami"> Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4 </ref> Sodium ions then bind to NTCP, favoring the open-pore state and also allowing for the binding of another outer bile salt (Fig 5). The <scene name='95/952721/Mech_step_1/1'>protein is then reset</scene> and the process can then start again releasing the next inner bile salt with the translocation of the sodium ions into the cytoplasm. |
| + | |||
| + | == HBV Binding and Infection== | ||
| + | NTCP is the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] into the liver for HBV. <Ref name = "Asami"/> The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through a <scene name='95/952721/Hbv_patch/2'>hydrophobic patch</scene> containing <b><font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> Another hydrophobic patch consisting of <b><font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that don't inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> | ||
| + | |||
| + | The exact mechanism by which NTCP mediates viral internalization is still yet to be determined; however, current studies speculate it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. It is currently unknown whether HBV also interacts with other receptors or host cell factors, but NTCP alone is not sufficient for infection. <Ref name = "Herrscher"/> | ||
== Medical Relevancy == | == Medical Relevancy == | ||
Revision as of 00:28, 7 April 2023
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ 6.0 6.1 6.2 Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4
- ↑ 7.0 7.1 7.2 7.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
- ↑ 8.0 8.1 Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259.
Student Contributors
- Isabelle White
- Lena Barko