We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1785
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
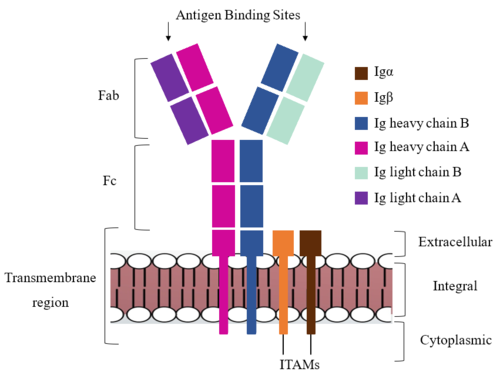
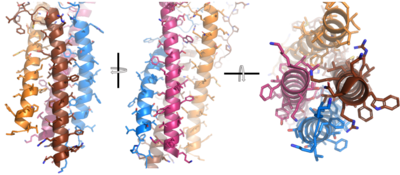
After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure 2) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. | After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure 2) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. | ||
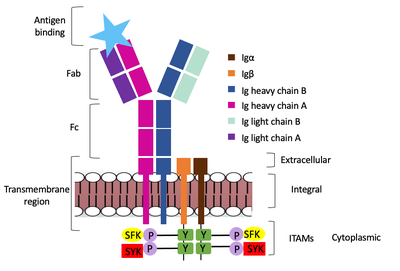
| - | Furthermore, both the Igα and Igβ chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain | + | Furthermore, both the Igα and Igβ chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an immuno-receptor tyrosine-based activation motif (ITAM) region to facilitate signal transduction (Figure 4). |
[[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | ||
{{Clear}} | {{Clear}} | ||
| + | |||
| + | Within the transmembrane region, heavy chains A and B associate asymmetrically to facilitate intracellular signaling cascades. The <scene name='95/952713/Trans_heavy/2'>transmembrane heavy chain interface</scene> allows them to pack together via Van der Waal contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on heavy chain A donates a hydrogen bond to Ser584 and to Ser588 on heavy chain B. This creates a bifurcated bond (blue link), essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the Igα and Igβ proteins w/in the BCR. | ||
===Fc Region=== | ===Fc Region=== | ||
| Line 31: | Line 33: | ||
<scene name='95/952715/Alpha_beta_heavy/2'>Alpha, Beta, and Heavy chain interactions</scene> help to stabilize and hold the heavy chains and Ig Alpha/Beta chains together in the extracellular portion of the intermembrane region. | <scene name='95/952715/Alpha_beta_heavy/2'>Alpha, Beta, and Heavy chain interactions</scene> help to stabilize and hold the heavy chains and Ig Alpha/Beta chains together in the extracellular portion of the intermembrane region. | ||
| - | <scene name='95/952713/Disulfides/4'> | + | Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, heavy chains A and B make a <scene name='95/952713/Disulfides/4'>disulfide bridge</scene> to stabilize the IgM-BCR and drive downstream signaling. |
To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of heavy chain A makes an asymmetrical association with constant region three of heavy chain B to create a <scene name='95/952713/Trans_heavy/5'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from heavy chain A donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on heavy chain B. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. | To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of heavy chain A makes an asymmetrical association with constant region three of heavy chain B to create a <scene name='95/952713/Trans_heavy/5'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from heavy chain A donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on heavy chain B. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. | ||
| - | |||
| - | <scene name='95/952713/Trans_heavy/2'>transmembrane heavy chain interface</scene> | ||
===Fab Region=== | ===Fab Region=== | ||
Revision as of 03:52, 7 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
Student Contributors
Detonyeá Dickson, Allison Goss, Jackson Payton