Sandbox Reserved 1786
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
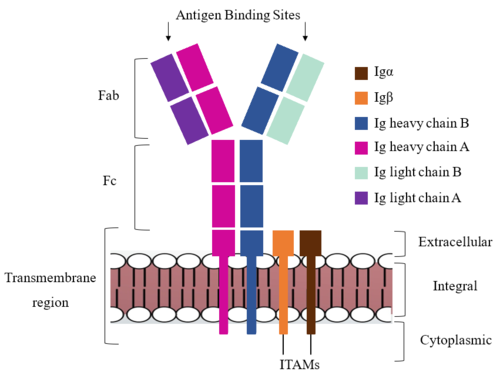
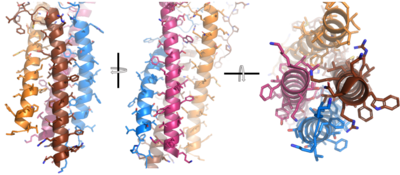
After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. (Tolar citation) The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure 2) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. (Dylke citation) | After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane. (Tolar citation) The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene> are primarily hydrophobic side chains that allow for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The 4 helices (Figure 2) are primarily held together through hydrophobic interactions; however, a a few polar residues are included on the interior of the helix structure which interact with a few polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. (Dylke citation) | ||
| + | |||
| + | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | ||
| + | {{Clear}} | ||
Within the transmembrane region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> associate (Figure 1) asymmetrically to facilitate intracellular signaling cascades. The <scene name='95/952713/Trans_heavy/2'>transmembrane heavy chain interface</scene> allows them to pack together via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on '''{{Font color|violet|heavy chain A}}''' donates a hydrogen bond to Ser584 and to Ser588 on <b><span class="text-blue">heavy chain B</span></b>. This creates a [https://en.wikipedia.org/wiki/Hydrogen_bond bifurcated hydrogen bond], essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains within the BCR (cite). | Within the transmembrane region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> associate (Figure 1) asymmetrically to facilitate intracellular signaling cascades. The <scene name='95/952713/Trans_heavy/2'>transmembrane heavy chain interface</scene> allows them to pack together via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on '''{{Font color|violet|heavy chain A}}''' donates a hydrogen bond to Ser584 and to Ser588 on <b><span class="text-blue">heavy chain B</span></b>. This creates a [https://en.wikipedia.org/wiki/Hydrogen_bond bifurcated hydrogen bond], essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains within the BCR (cite). | ||
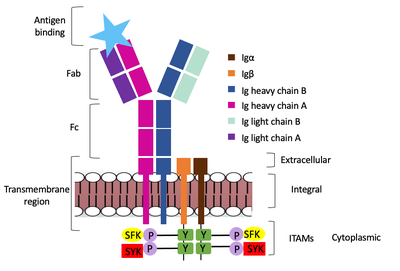
Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). (Ma citation) | Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). (Ma citation) | ||
| - | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' Pymol image of the integral helices in IgM BCR (PDB:7xq8) rotated on the x and y axes. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | ||
| - | {{Clear}} | ||
===Fc Region=== | ===Fc Region=== | ||
| - | The constant region of IgM is made up of the | + | The constant region of IgM is made up of the two <scene name='95/952715/Heavy_chain/1'>heavy chains</scene>. These heavy chains form a bridge to connect the Fab fragment, or variable region, to the transmembrane region. They also act as a wire to allow the variable region to send a cellular signal through to the intermembrane region once an antigen has been bound. |
Interactions between the <scene name='95/952715/Alpha_beta_heavy/2'>Igα, Igβ, and Heavy chains</scene> help to stabilize and hold the complex together in the extracellular portion of the transmembrane region. | Interactions between the <scene name='95/952715/Alpha_beta_heavy/2'>Igα, Igβ, and Heavy chains</scene> help to stabilize and hold the complex together in the extracellular portion of the transmembrane region. | ||
| Line 42: | Line 43: | ||
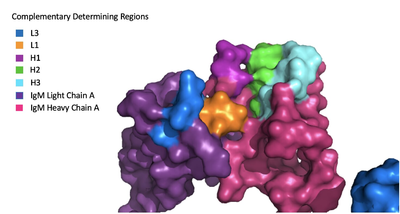
The IgM-BCR contains areas referred to as [https://en.wikipedia.org/wiki/Complementarity-determining_region complementary-determining regions](CDRs), which are where the antigen makes contact with the antibody on the Fab domain. Figure 2 depicts this as a surface representation given that the specific residues within the antigen-binding motif are unknown. | The IgM-BCR contains areas referred to as [https://en.wikipedia.org/wiki/Complementarity-determining_region complementary-determining regions](CDRs), which are where the antigen makes contact with the antibody on the Fab domain. Figure 2 depicts this as a surface representation given that the specific residues within the antigen-binding motif are unknown. | ||
| - | Due to the poor resolution of the Fab region, specific side chain interactions between the heavy and light chains have not been determined. It is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. The <scene name='95/952713/Heavy-light_chain_interface/1'>heavy-light chain interface</scene> shows how the four heavy and light chain β-sandwiches fit together. The Fab region heavy chains attach to the Fc region heavy chains | + | Due to the poor resolution of the Fab region, specific side chain interactions between the heavy and light chains have not been determined. It is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. The <scene name='95/952713/Heavy-light_chain_interface/1'>heavy-light chain interface</scene> shows how the four heavy and light chain β-sandwiches fit together. The Fab region heavy chains attach to the Fc region heavy chains, before continuing down into the intracellular domain to interact with the <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> subunits. The light chains however are only connected to the heavy chains within the Fab region, thus have no contact with the α/β heterodimer. |
[[Image:Igm_surface.png|400 px|left|thumb|'''Figure 3. Surface Representation of IgM Antibody Binding Pocket.''']] | [[Image:Igm_surface.png|400 px|left|thumb|'''Figure 3. Surface Representation of IgM Antibody Binding Pocket.''']] | ||
Revision as of 15:24, 7 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
Student Contributors
Detonyeá Dickson, Allison Goss, Jackson Payton