Sandbox Reserved 1786
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
=='''Structure'''== | =='''Structure'''== | ||
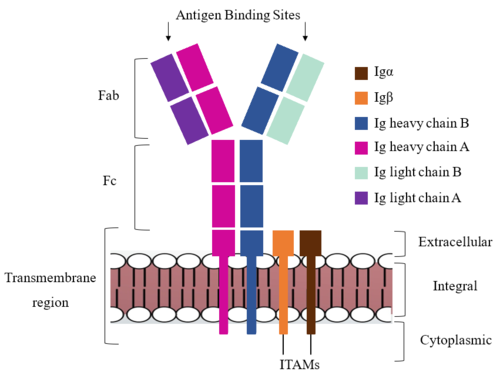
| - | The IgM BCR consists of six separate chains (Figure 1) that make up three main domains in the molecule. A depiction of the IgM <scene name='95/952714/Colored_by_domain/3'>colored by domain</scene> shows two heavy and two light chains that together form the <b><span class="text-cyan">Fab region</span></b>, or variable fragment at the top of the molecule where the antigen binding sites are located. The two heavy chains extend below the <b><span class="text-cyan">Fab region</span></b> through the <b><span class="text-purple">Fc region</span></b> and eventually connect to the Igα/β heterodimer to form the <b><span class="text-orange">transmembrane region</span></b> which anchors the overall complex to the B cell. The overall structure, expression, and function of the IgM BCR has been found to be strongly influenced by the <b><span class="text-orange">transmembrane region</span></b> in which Ig α/β interactions as a heterodimer influence cell surface expression, receptor assembly, and effective signal transduction (Tolar and Dylke citation). In each domain, interactions between individual chains are important to understand the complex as a whole. All future 3D depictions will be <scene name='95/952713/Colored_by_chain/ | + | The IgM BCR consists of six separate chains (Figure 1) that make up three main domains in the molecule. A depiction of the IgM <scene name='95/952714/Colored_by_domain/3'>colored by domain</scene> shows two heavy and two light chains that together form the <b><span class="text-cyan">Fab region</span></b>, or variable fragment at the top of the molecule where the antigen binding sites are located. The two heavy chains extend below the <b><span class="text-cyan">Fab region</span></b> through the <b><span class="text-purple">Fc region</span></b> and eventually connect to the Igα/β heterodimer to form the <b><span class="text-orange">transmembrane region</span></b> which anchors the overall complex to the B cell. The overall structure, expression, and function of the IgM BCR has been found to be strongly influenced by the <b><span class="text-orange">transmembrane region</span></b> in which Ig α/β interactions as a heterodimer influence cell surface expression, receptor assembly, and effective signal transduction (Tolar and Dylke citation). In each domain, interactions between individual chains are important to understand the complex as a whole. All future 3D depictions will be <scene name='95/952713/Colored_by_chain/7'>colored by chain</scene> as in Figure 1. |
[[Image:IgM_structure_overview_diagram.png|500 px|left|thumb|'''Figure 1. IgM BCR Structure Overview.''' Depiction of the IgM BCR expressed on the membrane of a B cell. Includes all major components including the α/β heterodimer, heavy and light chains, antigen binding sites, and the ITAM region for signal transduction.]] | [[Image:IgM_structure_overview_diagram.png|500 px|left|thumb|'''Figure 1. IgM BCR Structure Overview.''' Depiction of the IgM BCR expressed on the membrane of a B cell. Includes all major components including the α/β heterodimer, heavy and light chains, antigen binding sites, and the ITAM region for signal transduction.]] | ||
{{Clear}} | {{Clear}} | ||
| Line 52: | Line 52: | ||
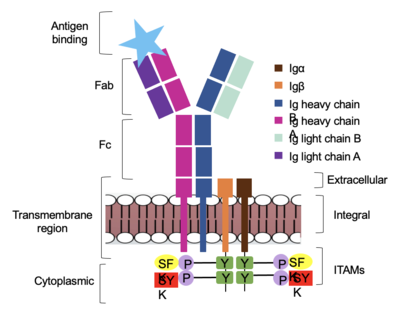
The diagram in Figure 4 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism (Ma). This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b>. In its “off” state, the constant region 4 of <b><span class="text-blue">heavy chain B</span></b> overlaps the extracellular components of <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b>. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] (observed here as the conserved tyrosine residues are phosphorylated) within the intracellular tails of <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> drives downstream kinase activity to continue to process of [https://en.wikipedia.org/wiki/Tyrosine-protein_kinase_SYK signal cascading]. | The diagram in Figure 4 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism (Ma). This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b>. In its “off” state, the constant region 4 of <b><span class="text-blue">heavy chain B</span></b> overlaps the extracellular components of <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b>. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] (observed here as the conserved tyrosine residues are phosphorylated) within the intracellular tails of <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> drives downstream kinase activity to continue to process of [https://en.wikipedia.org/wiki/Tyrosine-protein_kinase_SYK signal cascading]. | ||
| - | [[Image: | + | [[Image:Signal_binding.png|400 px|left|thumb|'''Figure 4. IgM Antibody Signal Transduction following Antigen Binding.''']] |
{{Clear}} | {{Clear}} | ||
Revision as of 16:14, 7 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
Student Contributors
DeTonyeá Dickson, Allison Goss, Jackson Payton