We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
=Introduction= | =Introduction= | ||
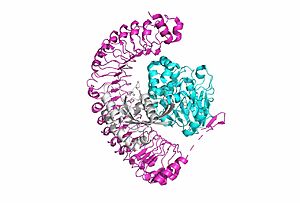
[[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | [[Image:SMP complex.jpg|300 px|right|thumb|'''Figure 1:'''Overall cartoon of SHOC2-PP1C-MRAS structure with SHOC2 in pink, PP1C in blue, and MRAs in white.</div></font>]] | ||
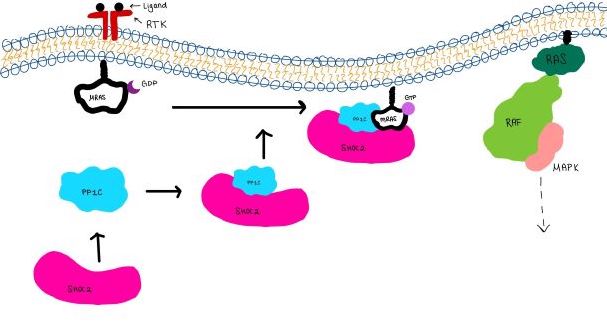
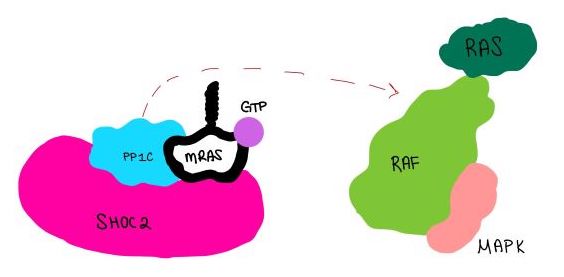
| - | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane bound MRAS to exchange GDP for GTP. From here the complex comes together | + | <scene name='95/952718/Zoom_out/1'>SHOC2-PP1C-MRAS</scene> (SMP) is a ternary holophosphotase complex formed by the individual proteins: SHOC2, PP1C, and MRAS. Formation of this complex begins with a signal binding to a receptor tyrosine kinase receptor(RTK). This causes membrane-bound MRAS to exchange GDP for GTP. From here the complex comes together in the plasma membrane. Its role in MAPK signaling is the dephosphorylation of the N-terminal phosphoserine (NTpS) on the RAF complex leading to further downstream signaling effects. |
=Overall Structure= | =Overall Structure= | ||
==SHOC2== | ==SHOC2== | ||
| - | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. | + | <scene name='95/952717/Shoc2/1'>SHOC2</scene> is a scaffold protein that is composed of 20 leucine-rich repeat domains that form a solenoid structure. The leucine rich region forms a concave hydrophobic core which is necessary for binding with PP1C and MRAS. SHOC2 is the crucial mediator for SHOC2-PP1C-MRAS complex formation. |
==PP1C== | ==PP1C== | ||
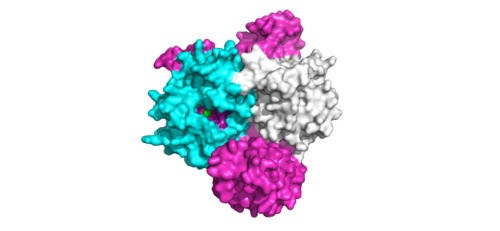
<scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the <scene name='95/952717/Pp1c_hydrophobic_patch/1'>hydrophobic active site</scene> on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. | ||
[[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 2:'''Active site of PP1C on SMP.</div></font>]] | ||
==MRAS== | ==MRAS== | ||
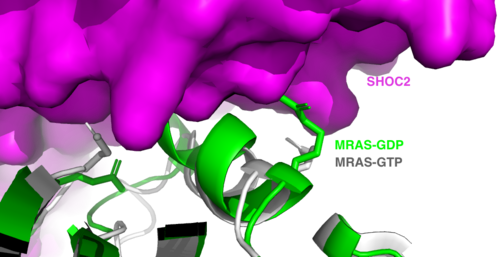
| - | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by | + | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>PMID:11756411</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. |
| + | |||
[[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | [[Image:Switches.png|500 px|thumb|'''Figure 2:'''Steric clashing of Switch I and II of GDP bound MRAS, in green, with the surface of SHOC2, in magenta. GTP-bound MRAS, in white, shows no steric clashing with SHOC2s surface.</div></font>]] | ||
Revision as of 16:15, 7 April 2023
| |||||||||||