We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
==PP1C== | ==PP1C== | ||
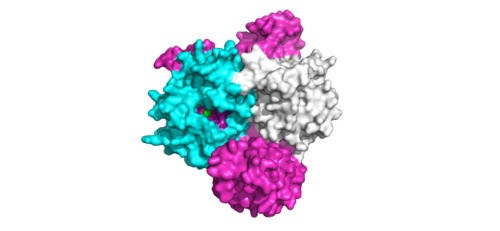
<scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the hydrophobic active site on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of N-terminal phosphoserine of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF <ref name="Hauseman" />. | <scene name='95/952717/Pp1c/1'>PP1C</scene> is a catalytic protein. After forming a ternary complex, the hydrophobic active site on the protein interacts with Raf to act as a phosphatase and dephosphorylate Ser 259. PP1C's active site is adjacent to a hydrophobic patch. It's theorized that the hydrophobic patch binds to the C-terminal of N-terminal phosphoserine of RAF, the target for dephosphorylation. PP1C can act as a phosphatase in the absence of SHOC2 but PP1C lasks intrinsic substrate selectively. So SMP complex formation is necessary for PP1C specificity to RAF <ref name="Hauseman" />. | ||
| - | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure | + | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 1:'''Active site of PP1C on SMP.</div></font>]] |
==MRAS== | ==MRAS== | ||
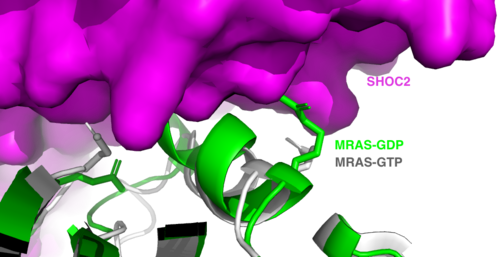
<scene name='95/952717/Mras/2'>MRAS</scene> is a membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. | <scene name='95/952717/Mras/2'>MRAS</scene> is a membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. | ||
| Line 30: | Line 30: | ||
The interactions between <scene name='95/952717/Mras_and_pp1c/1'>PP1C and MRAS</scene> are mediated by four main <scene name='95/952716/Mras_and_pp1c/1'>hydrogen bonds</scene>: R188-D48, M190-Q35, D197-H53, Q198-K36. It is unclear whether PP1C must bind to SHOC2 before MRAS binds or if PP1C and MRAS can bind to SHOC2 at the same time <ref name="Lavoie" />. | The interactions between <scene name='95/952717/Mras_and_pp1c/1'>PP1C and MRAS</scene> are mediated by four main <scene name='95/952716/Mras_and_pp1c/1'>hydrogen bonds</scene>: R188-D48, M190-Q35, D197-H53, Q198-K36. It is unclear whether PP1C must bind to SHOC2 before MRAS binds or if PP1C and MRAS can bind to SHOC2 at the same time <ref name="Lavoie" />. | ||
=Signaling Pathway= | =Signaling Pathway= | ||
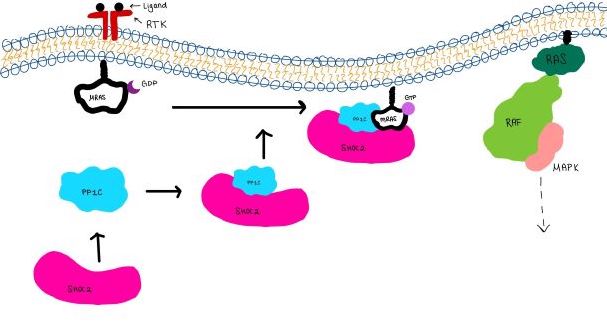
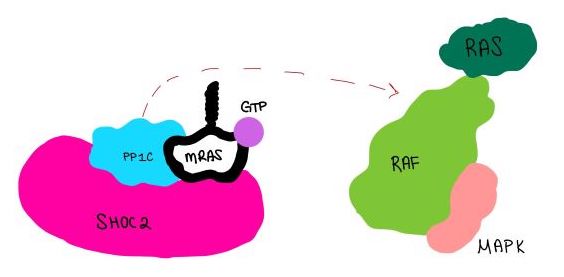
| - | The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the | + | The SMP signaling pathway begins with the formation of the SMP complex. Initially, a ligand must bind to a receptor tyrosine kinase. This signals SHOC2 to bind to PP1C forming a binary complex that then binds to the membrane bound MRAS. Some literature indicates that the three proteins bind at the same time but the order is largely unknown. Figure 2 shows the proteins binding one at a time. Once the SMP complex forms, its target is the NTpS also known as S259. The serine is directly dephosphorylated by PP1C by SHOC2 and MRAS increase its specificity for S259. |
Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling<ref name="Liau">PMID:35768504</ref>. | Mutations affecting SMP complex formation and stability have been shown to increase or decrease MAPK signaling. Increased stability of the complex increases MAPK signaling while decreased stability decreases signaling<ref name="Liau">PMID:35768504</ref>. | ||
| - | [[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure | + | [[Image:Signal_cascade_small.jpg|800 px|thumb|center|'''Figure 3:'''Signaling cascade is shown with SHOC2 in pink, PP1C in blue, and MRAs in white. ]] |
| - | [[Image:Dephosphorylation.jpg|600 px|thumb|center|'''Figure | + | [[Image:Dephosphorylation.jpg|600 px|thumb|center|'''Figure 4:'''PP1C dephosphorylates RAF protein at serine 259 ]] |
=Disease Relevance= | =Disease Relevance= | ||
Revision as of 17:09, 7 April 2023
| |||||||||||