We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1774
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
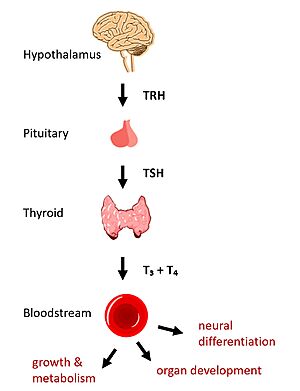
Dysregulation of TSHR can lead to disease. In [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease#:~:text=Graves'%20disease%20is%20an%20autoimmune,the%20way%20your%20heart%20beats. Grave's disease], antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism <ref name="Chu" />. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated <ref name="Brent" />. | Dysregulation of TSHR can lead to disease. In [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease#:~:text=Graves'%20disease%20is%20an%20autoimmune,the%20way%20your%20heart%20beats. Grave's disease], antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism <ref name="Chu" />. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated <ref name="Brent" />. | ||
| - | == | + | == Significance of TSHR Structure to Signaling Activation == |
| - | There are three main domains of the thyroid stimulating hormone receptor. First is the <scene name='95/952703/Tmd/9'>extracellular domain</scene>, which is concave in shape. It is also called the leucine rich region because it is made primarily of beta sheets which are rich in leucine <ref name="Kleinau">Kleinau G, Worth CL, Kreuchwig A, et al. Structural–Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work. Frontiers in Endocrinology. 2017;8. Accessed April 2, 2023. [https://doi.org/10.3389/fendo.2017.00086 DOI: 10.3389/fendo.2017.00086]</ref>. This domain also contains lysine residues which play a key role in TSH binding. Second is the <scene name='95/952703/Tmd/10'>transmembrane domain</scene> composed of seven alpha helices. The TMD undergoes a conformation change upon ligand binding that activates the intracellular GPCR signal cascade <ref name="Duan" />. The third region of the TSHR is the <scene name='95/952703/Tmd/11'>hinge region</scene> shown in orange. The hinge region controls the movement and stability of the TSHR. Specifically, the hinge region allows the receptor to undergo a conformation change which turns the signaling pathway on and off, the details of which are discussed in the proceeding section. | + | === Domains of TSHR Contribute to Signaling === |
| + | There are three main domains of the thyroid stimulating hormone receptor, each of which plays a distinct role in signaling activation. First is the <scene name='95/952703/Tmd/9'>extracellular domain</scene>, which is concave in shape. It is also called the leucine rich region because it is made primarily of beta sheets which are rich in leucine <ref name="Kleinau">Kleinau G, Worth CL, Kreuchwig A, et al. Structural–Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work. Frontiers in Endocrinology. 2017;8. Accessed April 2, 2023. [https://doi.org/10.3389/fendo.2017.00086 DOI: 10.3389/fendo.2017.00086]</ref>. This domain also contains lysine residues which play a key role in TSH binding. Second is the <scene name='95/952703/Tmd/10'>transmembrane domain</scene> composed of seven alpha helices. The TMD undergoes a conformation change upon ligand binding that activates the intracellular GPCR signal cascade <ref name="Duan" />. The third region of the TSHR is the <scene name='95/952703/Tmd/11'>hinge region</scene> shown in orange. The hinge region controls the movement and stability of the TSHR. Specifically, the hinge region allows the receptor to undergo a conformation change which turns the signaling pathway on and off, the details of which are discussed in the proceeding section. | ||
| - | + | === Hinge Motion Controls Signaling Activation === | |
| - | === Hinge Motion === | + | The TSHR exists in two states: <scene name='95/952702/Overlay/2'>active and inactive </scene>. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. Upward movement of the ECD into the active states pulls on the transmembrane helices to activate the G-protein, which in turns set off an intracellular signaling cascade that ends in transcriptional activation of thyroid hormones, including T3 and T4 (Fig. __). |
| - | + | ||
| - | + | The hinge motion of the TSHR is made possible by the hinge region structure. Deformation of the hinge region accommodates up-and-down rotation of the extracellular domain as a rigid body, which hinges approximately 55 degrees around an imaginary axis <ref name="Faust">PMID:35940205</ref> [[Image:TSHR_MorphBetterAngle.mp4|Figure 1]]. As the receptor transitions towards its active state, stretching of the hinge region leads to shifting of the transmembrane helices. Additionally, the hinge region itself serves as an agonist of TSHR activation through intermolecular interactions with the transmembrane domain in the active state. While switching between the active and inactive states is spontaneous, these hinge interactions favor the active conformation, as does binding of the TSH ligand <ref name="Faust">PMID:35940205</ref>. To fully understand these stabilizing interactions, a deeper discussion of hinge region anatomy is warranted. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | === Stabilizing Interactions in the Hinge === | + | === Stabilizing Interactions in the Hinge Favor Signaling Activation === |
| - | + | The hinge region can be subdivided into the <scene name='95/952702/Hinge_helix_intro/3'>hinge helix</scene>, which lies at the intersection of the extracellular and transmembrane domains; <scene name='95/952702/Helix_1_intro/5'>helix 1</scene>, which sticks up and serves as a binding platform for the TSH ligand; the <scene name='95/952702/Linker_intro/4'>linker</scene> region, which connects helix 1 with the p10 region; and the <scene name='95/952702/P10_intro/2'>p10</scene> region, a conserved 10-amino acid sequence which connects to transmembrane helix 7 and undergoes most of the deformation <ref name="Faust" /> <ref name="Duan">PMID:35940204</ref>. | |
Two key disulfide bridges within the hinge region help to maintain its structure and orientation <ref name="Duan" />. | Two key disulfide bridges within the hinge region help to maintain its structure and orientation <ref name="Duan" />. | ||
| Line 34: | Line 31: | ||
Interactions with the EC and TM domains help to stabilize the hinge in the upright, active conformation <ref name="Faust" />. | Interactions with the EC and TM domains help to stabilize the hinge in the upright, active conformation <ref name="Faust" />. | ||
#A <scene name='95/952702/Hydrophobic_interaction/2'>hydrophobic interaction</scene> occurs between Y279 in the hinge helix and I486 in EC loop region 1, which protrudes from the TM helices. | #A <scene name='95/952702/Hydrophobic_interaction/2'>hydrophobic interaction</scene> occurs between Y279 in the hinge helix and I486 in EC loop region 1, which protrudes from the TM helices. | ||
| - | #An <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> occurs between K660 in TM helix 7 and E409 in the p10 region. | + | #An <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> occurs between K660 in TM helix 7 and E409 in the p10 region. |
| + | |||
| + | Two observations help to explain how hinging of the extracellular domain can lead to signaling activation: | ||
| + | #Slinky-like deformation of the hinge region shifts the <scene name='95/952702/P10_movement/3'>N-terminus of the p10</scene> region 5 Angstroms over the course of the movement <ref name="Faust" />. | ||
| + | #Stretching of the hinge pulls on the linked helices in the transmembrane domain, shifting <scene name='95/952702/Helix7_movement/2'>helix 7</scene> about 4 Angstroms inward <ref name="Faust" />. | ||
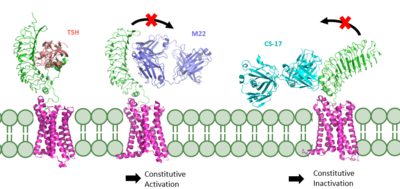
If these stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine overly stabilizes the <scene name='95/952702/Hydrophobic_interaction/2'>hydrophobic interaction</scene>, leading to overactivation <ref name="Faust" />. Contrastingly, TSHR underactivation results from disrupting the <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> with an E409A mutation, which is associated with diminished receptor activation and TSH potency <ref name="Faust" />. | If these stabilizing interactions are disrupted, TSHR function is affected. For instance, the mutation I496F has been observed to cause constitutive receptor activation and decreased sensitivity to the TSH ligand, suggesting that the bulkier phenylalanine overly stabilizes the <scene name='95/952702/Hydrophobic_interaction/2'>hydrophobic interaction</scene>, leading to overactivation <ref name="Faust" />. Contrastingly, TSHR underactivation results from disrupting the <scene name='95/952702/Ionic_interaction/3'>ionic interaction</scene> with an E409A mutation, which is associated with diminished receptor activation and TSH potency <ref name="Faust" />. | ||
| - | == Ligand Binding == | + | == Significance of TSHR Structure to Ligand Binding == |
| - | === Binding of Thyroid Stimulating Hormone to TSHR=== | + | === Binding of Thyroid Stimulating Hormone to TSHR === |
The thyroid stimulating hormone <scene name='95/952703/Tsh-ecd/3'>binds to the extracellular domain</scene> by complementary shape. The ECD is curved and compliments the curvature of TSH similar to how a baseball fits into a glove. There are also several key ionic interactions between the TSH and TSHR. The key ionic interactions occur in the <scene name='95/952703/Seatbelt/3'>seat belt region of TSH</scene> which is highlighted in yellow. The seatbelt region is located in the beta subunit of the TSH. <scene name='95/952703/Tsh-tshr_itxn-3/4'>The first ionic interaction</scene> is Glu118 from TSH and Lys58 from the ECD.<scene name='95/952703/Tsh-tshr_itxn-2/3'>The second interaction</scene> is between Asp111 from the TSH and Lys209 from the ECD. These interactions form salt bridges between the ECD and the TSH which allows for specificity of binding for TSH to TSHR <ref name="Duan" />,<ref name="Faust" />. | The thyroid stimulating hormone <scene name='95/952703/Tsh-ecd/3'>binds to the extracellular domain</scene> by complementary shape. The ECD is curved and compliments the curvature of TSH similar to how a baseball fits into a glove. There are also several key ionic interactions between the TSH and TSHR. The key ionic interactions occur in the <scene name='95/952703/Seatbelt/3'>seat belt region of TSH</scene> which is highlighted in yellow. The seatbelt region is located in the beta subunit of the TSH. <scene name='95/952703/Tsh-tshr_itxn-3/4'>The first ionic interaction</scene> is Glu118 from TSH and Lys58 from the ECD.<scene name='95/952703/Tsh-tshr_itxn-2/3'>The second interaction</scene> is between Asp111 from the TSH and Lys209 from the ECD. These interactions form salt bridges between the ECD and the TSH which allows for specificity of binding for TSH to TSHR <ref name="Duan" />,<ref name="Faust" />. | ||
Revision as of 01:19, 9 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||