We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1783
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Background == | == Background == | ||
| - | [[Image: | + | [[Image:Fig_1.png|400 px|right|thumb|'''Figure 1.''' NTCP structure with both Na+ ions and bile salts bound with the NTCP molecule shown in light blue, the two Na+ ions shown in purple spheres, and the bile salts shown in sticks as dark blue. [PDB file 7ZYI].]] |
| - | + | ||
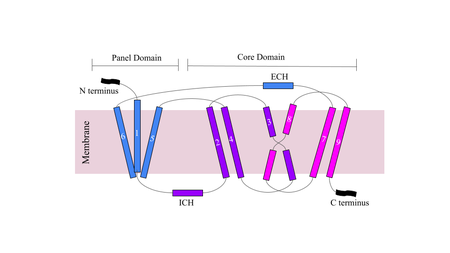
| - | NTCP | + | *Sodium taurocholate co-transporting polypeptide (NTCP) is a sodium-dependent transporter that is responsible for the transportation of bile salts from the blood into epithelial liver cells. <Ref name="Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> NTCP is a secondary active transport molecule that couples the thermodynamically favorable movement of Na+ ions with the unfavorable transport of bile salts into the cell (Fig. 1). |
| + | |||
| + | *The bile salts transported by NTCP in the gastrointestinal tract are involved in digestion, nutrient absorption, fat breakdown, and lipid soluble nutrient transport. <Ref> Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. [https://doi.org/10.1016/j.cis.2010.12.002 DOI: 10.1016/j.cis.2010.12.002]. </Ref> NTCP is found within the basolateral membrane hepatocytes. <Ref name="Asami"> Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. [https://dx.doi.org/10.1038/s41586-022-04845-4 DOI: 10.1038/s41586-022-04845-4]. </Ref> The uptake of bile salts into the liver also allow for drugs and fat soluble vitamins to be both absorbed and excreted in the small intestine. NTCP also acts as a receptor for Hepatitis B virus (HBV) and Hepatitis D virus (HDV) which infect human livers through endocytosis when bound. The myristoylated (myr) <scene name='95/952711/Pres1_binding_area_on_ntcp/3'>pre-S1</scene> domain of HBV, specifically residues 8-17, is critical for its binding to NTCP which halts the uptake of bile salts, indicating that HBV/HDV bind to NTCP at the same site as bile salts. <ref name="Goutam"/> | ||
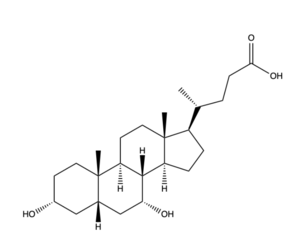
[[Image:Bile Salt structure.png|300 px|left|thumb|'''Figure 2.''' Bile Salt Structure.]] | [[Image:Bile Salt structure.png|300 px|left|thumb|'''Figure 2.''' Bile Salt Structure.]] | ||
| Line 19: | Line 20: | ||
=== Binding Pocket === | === Binding Pocket === | ||
| - | + | *<scene name='95/952711/Sodium_residue_zoom_in_nctp/1'>Sodium binds to residues 84-87 and 157-165 within NTCP </scene>. The binding pocket forms a tunnel structure within NTCP at the interface of two domains that connects the external cytoplasm of the hepatocyte to the basolateral membrane. The face of the tunnel where the bile salts bind is lined with hydrophilic residues, whereas the opposite face of the transmembrane helices is hydrophobic, making the tunnel amphipathic. <Ref name="Liu"> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). [https://doi.org/10.1038/s41422-022-00680-4 DOI: 10.1038/s41422-022-00680-4]. </Ref> The hydrophilic tunnel allows hydrophilic bile salts and sodium ions to be transported across the hydrophobic cell membrane. In the <scene name='95/952710/Tunnel_front/1'>outward facing state</scene> with no bile salt bound to the tunnel, it forms a hollow hole in the middle of the structure. When bile salts bind within, these bile salts completely occlude the <scene name='95/952710/Bile_bound_to_tunnel/2'>tunnel</scene>. | |
| - | [[Image:Patches.png|300 px|left|thumb|'''Figure 2.''' Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. PDB file 7ZYI.]] | ||
| - | + | [[Image:Patches.png|300 px|left|thumb|'''Figure 2.''' Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. [PDB file 7ZYI].]] | |
=== Conformation Change === | === Conformation Change === | ||
| Line 39: | Line 39: | ||
== Significance == | == Significance == | ||
| - | NTCP is a key player in the absorption and digestion of fats and fat-soluble vitamins in the body, as well as the synthesis of bile within the liver. The uptake of bile salts, transcriptional and post-transcriptional, are signaling molecules for the liver and intestine. Bile salt, the transporting molecule of NTCP, aids in the absorption of lipophilic nutrients and vitamins in the small intestine. Additionally, bile salt plays a role in the endocrine system, excretion of toxins, and cholesterol maintenance. | + | NTCP is a key player in the absorption and digestion of fats and fat-soluble vitamins in the body, as well as the synthesis of bile within the liver. The uptake of bile salts, transcriptional and post-transcriptional, are signaling molecules for the liver and intestine. Bile salt, the transporting molecule of NTCP, aids in the absorption of lipophilic nutrients and vitamins in the small intestine. Additionally, bile salt plays a role in the endocrine system, excretion of toxins, and cholesterol maintenance. <ref name="Goutam"/>. Thus, the significance of NTCP lies with the importance of bile salts; NTCP is necessary in maintaining bile salt levels and thus necessary in maintaining the aforementioned biological processes. |
=== Bile Salt Uptake === | === Bile Salt Uptake === | ||
| Line 45: | Line 45: | ||
Cirrhosis, commonly known as the last stage of liver disease, is due to a deficiency in the bile acid supply in the liver. Bile acid pools, as defined by Vlahcevic, are the negatively-charged precursors to bile and often are conjugated to positively-charged bile salts. This is attributed either a decrease in the production of bile salts or a large increase in the excretion of this molecule out of the cell. https://www.gastrojournal.org/article/S0016-5085(71)80053-7/pdf Cirrhosis causes symptoms such as jaundice, abdominal pain and swelling, and swelling of the legs and feet. A patient who has type two diabetes, men, and those who have or do abuse alcohol are also more sustainable to developing Cirrhosis. This diagnosis can lead to liver failure and other life-threatening diseases, making bile salt uptake essential to liver function. https://www.niddk.nih.gov/health-information/liver-disease/cirrhosis/definition-facts | Cirrhosis, commonly known as the last stage of liver disease, is due to a deficiency in the bile acid supply in the liver. Bile acid pools, as defined by Vlahcevic, are the negatively-charged precursors to bile and often are conjugated to positively-charged bile salts. This is attributed either a decrease in the production of bile salts or a large increase in the excretion of this molecule out of the cell. https://www.gastrojournal.org/article/S0016-5085(71)80053-7/pdf Cirrhosis causes symptoms such as jaundice, abdominal pain and swelling, and swelling of the legs and feet. A patient who has type two diabetes, men, and those who have or do abuse alcohol are also more sustainable to developing Cirrhosis. This diagnosis can lead to liver failure and other life-threatening diseases, making bile salt uptake essential to liver function. https://www.niddk.nih.gov/health-information/liver-disease/cirrhosis/definition-facts | ||
| - | === HBV/HDV === | ||
| - | |||
| - | Hepatitis B and D both rely on NTCP to bind and infect the human liver. HBV/HDV uses a the same patches as bile salt uptake within NTCP to infect the liver through endocytosis. The myristoylated (myr) pre-S1 domain of HBV is critical for its binding within the protein. Residues 8-17 within the <scene name='95/952711/Pres1_binding_area_on_ntcp/3'>pre-S1</scene> domain were found to be most important in pre-S1 binding to NTCP. HBV/HDV binding halts the uptake of bile salt, indicating that the tunnel formed within NTCP allows the uptake of bile salts and may also mediate the binding of HBV/HDV. [[https://www.nature.com/articles/s41586-022-04845-4]] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 19:52, 10 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Peptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ 4.0 4.1 Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, DOI: 10.1016/j.xinn.2022.100294.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.
![Figure 1. NTCP structure with both Na+ ions and bile salts bound with the NTCP molecule shown in light blue, the two Na+ ions shown in purple spheres, and the bile salts shown in sticks as dark blue. [PDB file 7ZYI].](/wiki/images/thumb/3/36/Fig_1.png/400px-Fig_1.png)
![Figure 2. Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. [PDB file 7ZYI].](/wiki/images/thumb/8/8c/Patches.png/300px-Patches.png)