Background
![Figure 1. NTCP structure with both Na+ ions and bile salts bound with the NTCP molecule shown in light blue, the two Na+ ions shown in purple spheres, and the bile salts shown in sticks as dark blue. [PDB file 7ZYI].](/wiki/images/thumb/3/36/Fig_1.png/400px-Fig_1.png)
Figure 1. NTCP structure with both Na+ ions and bile salts bound with the NTCP molecule shown in light blue, the two Na+ ions shown in purple spheres, and the bile salts shown in sticks as dark blue. [PDB file 7ZYI].
- Sodium taurocholate co-transporting polypeptide (NTCP) is a sodium-dependent transporter that is responsible for the transportation of bile salts from the blood into epithelial liver cells. [1] NTCP is a secondary active transport molecule that couples the thermodynamically favorable movement of Na+ ions with the unfavorable transport of bile salts into the cell (Fig. 1).
- The bile salts transported by NTCP in the gastrointestinal tract are involved in digestion, nutrient absorption, fat breakdown, and lipid soluble nutrient transport. [2] NTCP is found within the basolateral membrane hepatocytes. [3] The uptake of bile salts into the liver also allow for drugs and fat soluble vitamins to be both absorbed and excreted in the small intestine. NTCP also acts as a receptor for Hepatitis B virus (HBV) and Hepatitis D virus (HDV) which infect human livers through endocytosis when bound. The myristoylated (myr) domain of HBV, specifically residues 8-17, is critical for its binding to NTCP which halts the uptake of bile salts, indicating that HBV/HDV bind to NTCP at the same site as bile salts. [1]
Figure 2. Bile Salt Structure.
Structural Overview
NTCP has 9 transmembrane alpha helices (TM) that form the protein, with an extracellular N-terminus and intracellular C-terminus. NTCP has two domains within the protein, , made up of TM1, TM5, and TM6, and , made up of TM2-4 and TM7-9. An interesting feature of NTCP is the cross of TM3 and TM8 that form an within the protein that is used in the conformational change of NTCP. The two domains are essential to the conformation change of NTCP to bind bile salts [4].
There are two significant patches in the NTCP structure that facilitate ligand binding. Residues 84-87 of NTCP are Patch 1, which are located on the TM2-TM3 loop in the core domain. This patch is also considered the extracellular region of the binding tunnel within NTCP. Residues 157-165 of NTCP are associated with Patch 2. They are located on the N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in the extracellular region of the binding tunnel. These residues' importance was determined through mutations of these residues and examined through pull-down assays [3]. The pre-S1 domain of HBV/HDV binds to the patches on NTCP in order to transport the virus from the exterior of NTCP to the interior binding tunnel to infect human liver cells.
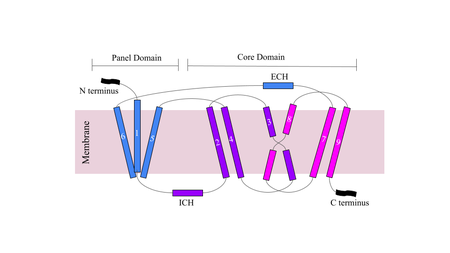
Figure 3. Cartoon of NTCP topology.
Binding Pocket
- . The binding pocket forms a tunnel structure within NTCP at the interface of two domains that connects the external cytoplasm of the hepatocyte to the basolateral membrane. The face of the tunnel where the bile salts bind is lined with hydrophilic residues, whereas the opposite face of the transmembrane helices is hydrophobic, making the tunnel amphipathic. [5] The hydrophilic tunnel allows hydrophilic bile salts and sodium ions to be transported across the hydrophobic cell membrane. In the with no bile salt bound to the tunnel, it forms a hollow hole in the middle of the structure. When bile salts bind within, these bile salts completely occlude the .
![Figure 2. Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. [PDB file 7ZYI].](/wiki/images/thumb/8/8c/Patches.png/300px-Patches.png)
Figure 2. Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. [PDB file 7ZYI].
Conformation Change
The conformational change of NTCP's core domain helices are essential to bile salt binding and uptake. Helices 3 and 8 [insert X domain highlight here] are the main structural components of the conformational change, as the X motif has highly conserved polar residue motifs that reside near the bile salt transport sites.. The conformational change is energized by the movement of Na+ down its concentration gradient. Before bile salt can bind, the pore in which salt binds must be . Conserved glycine and proline residues act as hinges in the connecting short loops, intracellular α-helices, and extracellular α-helices of NTCP to facilitate the movement of the core and panel domain to allow for a conformational change. The pore is flipped toward the outer membrane to allow for bile salt binding by exposing the Na+ binding sites and the X motif within NTCP. Once , the pore is , and bile salt is able to be released into the cell, past the inner membrane.
Mechanism

Figure 4. Bile Salt Uptake Mechanism.
The NTCP protein goes through a conformational change when assisting in the uptake of bile salt into the cell. This is accomplished through the opening of a wide transmembrane pore, creating a transport pathway for bile salts. The mechanism includes two metal ions that allow for residue stabilization when going through the conformational change. Binding of the preS1 region of the HBV/HDV virus blocks any subsequent bile salt uptake. Thus, preS1 binding blocks the conformational change and entry of any salts into the cell. Residues 8-17 of preS1 are critical for NTCP:pres1 binding. Patch 1 and Patch 2 (external) residues interact with residues 8-17 of preS1 to facilitate binding.
Significance
NTCP is a key player in the absorption and digestion of fats and fat-soluble vitamins in the body, as well as the synthesis of bile within the liver. The uptake of bile salts, transcriptional and post-transcriptional, are signaling molecules for the liver and intestine. Bile salt, the transporting molecule of NTCP, aids in the absorption of lipophilic nutrients and vitamins in the small intestine. Additionally, bile salt plays a role in the endocrine system, excretion of toxins, and cholesterol maintenance. [1]. Thus, the significance of NTCP lies with the importance of bile salts; NTCP is necessary in maintaining bile salt levels and thus necessary in maintaining the aforementioned biological processes.
Bile Salt Uptake
Cirrhosis, commonly known as the last stage of liver disease, is due to a deficiency in the bile acid supply in the liver. Bile acid pools, as defined by Vlahcevic, are the negatively-charged precursors to bile and often are conjugated to positively-charged bile salts. This is attributed either a decrease in the production of bile salts or a large increase in the excretion of this molecule out of the cell. [6] Cirrhosis causes symptoms such as jaundice, abdominal pain and swelling, and swelling of the legs and feet. A patient who has type two diabetes, men, and those who have or do abuse alcohol are also more sustainable to developing Cirrhosis. This diagnosis can lead to liver failure and other life-threatening diseases, making bile salt uptake essential to liver function.
![Figure 1. NTCP structure with both Na+ ions and bile salts bound with the NTCP molecule shown in light blue, the two Na+ ions shown in purple spheres, and the bile salts shown in sticks as dark blue. [PDB file 7ZYI].](/wiki/images/thumb/3/36/Fig_1.png/400px-Fig_1.png)


![Figure 2. Surface representation of the NTCP molecule with both patches shown. Patch 1 can be seen on the left side of the molecule, whereas patch 2 is located on the right side within the binding tunnel. [PDB file 7ZYI].](/wiki/images/thumb/8/8c/Patches.png/300px-Patches.png)

