Sandbox Reserved 1781
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
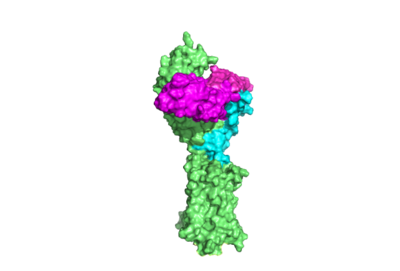
[[Image:surface TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, and thyrotropin bound in magenta.]] | [[Image:surface TSHR.png|400 px|right|thumb|Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, and thyrotropin bound in magenta.]] | ||
[https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to activity of thyroid cells as well as metabolic processes and oxygen consumption<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. Misregulation of thyroid hormone levels is the cause of many disorders related to [https://www.endocrineweb.com/conditions/thyroid/hyperthyroidism-vs-hypothyroidism hypo- or hyperthyroidism]. Thus, understanding the signaling of synthesis and release of these hormones will have applications in treating [https://www.hopkinsmedicine.org/health/conditions-and-diseases/disorders-of-the-thyroid thyroid hormone disorders]<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone (TSH), which | [https://en.wikipedia.org/wiki/Thyroid_hormones Thyroid hormones] exercise essential functions related to activity of thyroid cells as well as metabolic processes and oxygen consumption<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. Misregulation of thyroid hormone levels is the cause of many disorders related to [https://www.endocrineweb.com/conditions/thyroid/hyperthyroidism-vs-hypothyroidism hypo- or hyperthyroidism]. Thus, understanding the signaling of synthesis and release of these hormones will have applications in treating [https://www.hopkinsmedicine.org/health/conditions-and-diseases/disorders-of-the-thyroid thyroid hormone disorders]<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone (TSH), which | ||
| - | is released by the anterior pituitary gland<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The release of TSH from the anterior pituitary is regulated by thyroid-releasing hormone (TRH) which is released by the hypothalamus. When stimulated by TRH, the anterior pituitary releases TSH. When stimulated by TSH, the thyroid gland will produce and release the the thyroid hormones T4 and T3. T3 is the "active form" of the hormone, however it accounts for only 20% of the thyroid hormone that is released after stimulus by TSH. The T4 that predominates in release from the thyroid will be converted to T3 in the bloodstream. High levels of T3 and T4 can negatively regulate the release of TSH from the anterior pituitary, constituting a negative feedback loop <ref name="Pirahanchi et al.">Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/</ref>. The thyrotropin receptor <scene name='95/952709/Initial_scene_with_edited_7utz/2'>(TSHR)</scene> is a [https://www.nature.com/scitable/topicpage/gpcr-14047471/ G-protein coupled receptor] | + | is released by the anterior pituitary gland<ref name="Yen">Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.</ref>. The release of TSH from the anterior pituitary is regulated by thyroid-releasing hormone (TRH) which is released by the hypothalamus. When stimulated by TRH, the anterior pituitary releases TSH. When stimulated by TSH, the thyroid gland will produce and release the the thyroid hormones T4 and T3. T3 is the "active form" of the hormone, however it accounts for only 20% of the thyroid hormone that is released after stimulus by TSH. The T4 that predominates in release from the thyroid will be converted to T3 in the bloodstream. High levels of T3 and T4 can negatively regulate the release of TSH from the anterior pituitary, constituting a negative feedback loop <ref name="Pirahanchi et al.">Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/</ref>. The thyrotropin receptor <scene name='95/952709/Initial_scene_with_edited_7utz/2'>(TSHR)</scene> is a [https://www.nature.com/scitable/topicpage/gpcr-14047471/ G-protein coupled receptor] on the surface the thyroid gland cells. TSHR is responsible for binding TSH and transduces signal to initiate synthesis and release of thyroid hormones. In addition to TSH, autoantibodies may also bind to TSHR causing inhibition or activation of its desired function. (Figure 1)<ref name="Duan et al.">PMID:35940204</ref><ref name="Kohn et al.">Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.</ref> |
== Structure == | == Structure == | ||
===Overview=== | ===Overview=== | ||
| - | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. When thyrotropin | + | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. This <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the extracellular domain to the intracellular domain <ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. When thyrotropin binds, it causes a conformational change in the ECD that is transduced through the transmembrane helices. In the active state, the ECD is in the "up" position, while in the inactive state, the ECD is in the "down" state, closer to the cell membrane. A "push-pull" mechanism is proposed to be responsible for the ECD's conformational change between active and inactive states. In the "push" model, TSH binds to the receptor and sterically clashes with the cellular membrane forcing the ECD up away from the membrane. In the pull model, a short alpha helix interacts with TSH to pull the ECD up. The active (up) form of the ECD causes a conformation shift in the TMD which causes differential interactions with a heterotrimeric <scene name='95/952709/G_protein/2'>G-protein</scene>, initiating intracellular signaling. |
===Hinge Region and P10 Peptide=== | ===Hinge Region and P10 Peptide=== | ||
Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the ECD to the 7TMD. Also, this region, has been found to have impact on the binding potency of TSH as well as intracellular cyclic adenosine monophosphate (cAMP) levels, which are partially mediated by the activation of the GPCR. Several features of this region have been found to be crucial to the potent activation of of the TSHR by TSH.<ref name="Mizutori et al.">Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407</ref>. The most important feature of the hinge region is the interaction of the <scene name='95/952709/Hinge_helix_rotation/1'>hinge helix</scene> with the <scene name='95/952709/P10_peptide_region/2'>p10 peptide</scene> through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1)<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. These disulfides are critical as they link the ECD, where thyrotropin binds, to the TMD, whose conformational changes directly mediate the activation of the receptor's complementary G-protein. Movement of the ECD, caused by TSH binding, will cause rotation of the hinge helix and subsequent movement of the p10 peptide leading to movement of the transmembrane helices which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing Y385 and D386. Y385 is buried into a hydrophobic region of TSH while D386 forms a salt bride with R386 of the hormone. Together, <scene name='95/952709/Binding_interactions_hinge/1'>these interactions</scene> assist in facilitating the stable binding of TSH to the TSHR <ref name="Duan et al.">PMID:35940204</ref>. This being said, it is important to acknowledge, the hinge region itself is not required for the activation of the receptor<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. | Several parts of TSHR are very important for the functioning of TSH signaling. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the ECD to the 7TMD. Also, this region, has been found to have impact on the binding potency of TSH as well as intracellular cyclic adenosine monophosphate (cAMP) levels, which are partially mediated by the activation of the GPCR. Several features of this region have been found to be crucial to the potent activation of of the TSHR by TSH.<ref name="Mizutori et al.">Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407</ref>. The most important feature of the hinge region is the interaction of the <scene name='95/952709/Hinge_helix_rotation/1'>hinge helix</scene> with the <scene name='95/952709/P10_peptide_region/2'>p10 peptide</scene> through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1)<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. These disulfides are critical as they link the ECD, where thyrotropin binds, to the TMD, whose conformational changes directly mediate the activation of the receptor's complementary G-protein. Movement of the ECD, caused by TSH binding, will cause rotation of the hinge helix and subsequent movement of the p10 peptide leading to movement of the transmembrane helices which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing Y385 and D386. Y385 is buried into a hydrophobic region of TSH while D386 forms a salt bride with R386 of the hormone. Together, <scene name='95/952709/Binding_interactions_hinge/1'>these interactions</scene> assist in facilitating the stable binding of TSH to the TSHR <ref name="Duan et al.">PMID:35940204</ref>. This being said, it is important to acknowledge, the hinge region itself is not required for the activation of the receptor<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. | ||
===7 Transmembrane Helices=== | ===7 Transmembrane Helices=== | ||
| - | The | + | The ECD of TSHR is anchored to the membrane through seven transmembrane helices (7TMD), characteristic of GPCRs. Conformational changes in the 7TMD are responsible for intracellular signaling<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. Specifically, changes to this region are mediated by movements in the afforementioned p10 peptide. When the hinge helix rotates, it causes p10 peptide displacement that allows the <scene name='95/952709/Helix_7_of_7tmd/2'>seventh helix</scene> of the transmembrane domain to migrate towards the center of the 7TMD to increase van der waals contacts. In addition, lysine 660 of TM7 forms an <scene name='95/952709/Helix_7_and__p10_interaction/6'>ionic interaction</scene> with glutamate 409 of the p10 region. This interaction assists in the stabilization of the active state of 7TMD. In addition, the movement of the hinge helix has been found to bee associated with movement of a tyrosine residue relative to and isoleucine residue on the neighboring <scene name='95/952709/Hinge_helix_ecl1/3'>extracellular loop 1 (ECL1) helix</scene>. The identity of these residues has been found to be an important predictor in the activation of the thyrotropin receptor. For instance, structurally guided mutagenic studies have shown that the mutation of the isoleucine to a more sizeable phenylalanine decreases TSH signaling potency<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Vlaeminck-Guillem et al.">Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816</ref>.In addition to these conformational changes, the sixth transmembrane helix of TSHR is also moved outward from the center of the 7TMD to facilitate alpha helix 5 of the alpha subunit of the G protein, which is the domain that becomes activated<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Goricanec et al.">Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113 </ref>. |
| + | |||
== Relevance == | == Relevance == | ||
<scene name='95/952709/Interactions_with_thyrotropin/1'>Glu 98 and Asp 91</scene> | <scene name='95/952709/Interactions_with_thyrotropin/1'>Glu 98 and Asp 91</scene> | ||
Revision as of 20:27, 10 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ 3.0 3.1 3.2 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/
- ↑ 5.0 5.1 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 7.0 7.1 Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 9.0 9.1 9.2 9.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113