Introduction
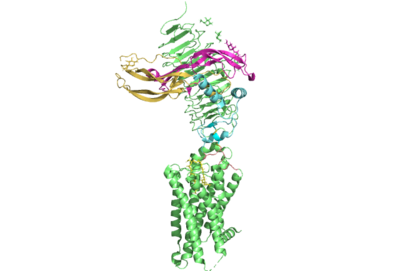
Figure 1. TSHR with TSH bound. The extracellular and transmembrane domains of the GPCR are shown in green, the hinge region in cyan, the P10 peptide in pink, and thyrotropin bound in pink and yellow.
Thyroid hormones exercise essential functions related to thymocyte activity as well as metabolic processes and oxygen consumption. Misregulation of thyroid hormones is the cause of many disorders related to hypo- or hyperthyroidism. Thus, understanding the signaling of synthesis and release of these hormones is essential to the development therapeutic drugs to combat specific thyroid hormone disorders[1]. The initiation of the synthesis and release of these hormones is caused by the glycoprotein, thyroid stimulating hormone, commonly referred to as TSH or thyrotropin. The thyrotropin receptor is a G-protein coupled receptor that binds TSH and transduces signal to initiate synthesis and release of thyroid hormones. It is important to note that autoantibodies may also bind to this receptor causing inhibition or activation of its desired function. (Figure 1)[2][3]
Structure
Overview
The thyrotropin receptor has an extracellular domain (ECD) that is composed of a as well as a hinge region. This links the ECD to the seven transmembrane helices , which span from the extracellular domain to the intracellular domain [4]. When thyrotropin or an autoantibody binds, it causes a conformational change in the receptor through the transmembrane helices. This causes the thyrotropin receptor to interact differently with its respective when in the active and inactive states.
Leucine Rich Region
The Leucine Rich region (LRRD) is part of the of TSHR and contains . A unique feature of this region is that it is composed entirely of β-pleated sheets. These β-pleated sheets of the LRRD provides a concave binding surface for TSH, including [2]. These interact with in the seatbelt region of TSH forming a salt bridge and initiating the conformational change by pulling on the hinge region of the receptor [5]. This interaction is specific to TSH and TSHR. When other agonists or antagonists bind to the receptor, the change in conformation is a result of different residues interacting, as explained later in the page. The Leucine residues in the LRRD determine ECD folding and which residues are located on the exterior protein and interacting with ligands.
Hinge Region and P10 Peptide
Several parts of TSHR are very important for the functioning of TSH signaling. The is a scaffold for the attachment of the ECD to the 7TMD. Also, this region, has been found to have impact on the binding potency of TSH as well as intracellular cyclic adenosine monophosphate (cAMP) levels, which are partially mediated by the activation of the GPCR. Several features of this region have been found to be crucial to the potent activation of of the TSHR by TSH.[6]. The most important feature of the hinge region is the interaction of the with the through disulfides. The p10 peptide is a conserved sequence that spans from the last beta sheet of the LRRD to the first transmembrane helix (TM1)[7]. These disulfides are critical as they link the ECD, where thyrotropin binds, to the TMD, whose conformational changes directly mediate the activation of the receptor's complementary G-protein. Movement of the ECD, caused by TSH binding, will cause rotation of the hinge helix and subsequent movement of the p10 peptide leading to movement of the transmembrane helices which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing Y385 and D386. Y385 is buried into a hydrophobic region of TSH while D386 forms a salt bride with R386 of the hormone. Together, assist in facilitating the stable binding of TSH to the TSHR [2]. This being said, it is important to acknowledge, the hinge region itself is not required for the activation of the receptor. In many of the aforementioned misregulations of thyroid hormone release, auto-antibodies are responsible. These auto-antibodies bind to the receptor through alternative interactions which cause conformational changes to the 7TMD without any need for a conformational change or extensive interactions with the hinge region[7].
7 Transmembrane Helices
The thyrotropin receptor is anchored to the membrane through seven transmembrane helices which is characteristic of GPCRs. Conformational changes in this region of the receptor are responsible for the activation of associated G protein[4]. In thyrotropin binding, changes to this region are mediated by movements in the afforementioned p10 peptide. When the hinge helix rotates, it causes p10 peptide displacement that allows the of the transmembrane domain to migrate towards the center of the 7TMD to increase van der waals contacts. In addition, lysine 660 of TM7 forms an with glutamate 409 of the p10 region. This interaction assists in the stabilization of the active state of 7TMD. In addition, the movement of the hinge helix has been found to bee associated with movement of a tyrosine residue relative to and isoleucine residue on the neighboring . The identity of these residues has been found to be an important predictor in the activation of the thyrotropin receptor. For instance, structurally guided mutagenic studies have shown that the mutation of the isoleucine to a more sizeable phenylalanine decreases TSH signaling potency[7][8].In addition to these conformational changes, the sixth transmembrane helix of TSHR is also moved outward from the center of the 7TMD to facilitate alpha helix 5 of the alpha subunit of the G protein, which is the domain that becomes activated[7][9].
Active and Inactive Form
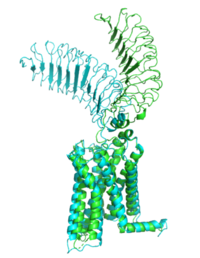
Figure 2: Inactive form of the thyrotropin receptor shown in blue. Active form of the thyrotropin receptor shown in green.
The TSHR protein exists in two states: active and inactive (Figure 2). The protrudes from the cell membrane into the space outside the cell. The contains 7 alpha helices that reside within the cell membrane. The exists when bound to the . One proposed mechanism for the transition from the active to inactive describes that in a natural state, the TSHR ECD can spontaneously transition to the up state, leading to constitutive activity. In this active state, TSH will bind and keep the active state in the up position because of clash with the cell membrane.[5] Conformational change of ECD allows for signal transduction through the TM and into the cell. The ECD rotates 55 degrees up in the active form. [5]
TSHR Agonists and Antagonists
Chemical agonists are found in many living systems and serve as a way to activate receptors or pathways that are necessary for a wide array of biological processes. Chemical antagonists block or inhibit biological processes. Different types of agonists/antagonists exist within the body including hormones, antibodies, and neurotransmitters. The body naturally produces autoantibodies that can act as agonists and mimic the activating mechanism of the natural hormone. Isolating these antibodies in patients with diseases can lead researchers to uncover the mechanism of binding for the receptor.
M22 Agonist and Grave's Disease
is a
monoclonal antibody that was isolated from a patient with
Graves' Disease. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease
effects 1 in 100 Americans and especially women or people older than 30 years of age. The binding of to results in the receptor remaining in its active conformation. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity.
[10]. The M22
autoantibody activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR because it is a potent activator for TSHR.
[5] Although M22 binds in a similar manner to TSH, M22 does not interact with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.
[5] This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation.
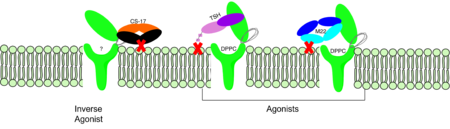
Figure 3: Agonist and antagonist drugs for activating or inactivating the TSHR protein. Here the membrane clashes are demonstrated on TSHR with different agonists attached. CS-17 is orange, TSH is purple, and M22 is blue in the figure. The TSHR protein is green and embedded in the protein.
CS-17 Inverse Agonist
is a monoclonal antibody that acts as an inverse agonist for TSHR constitutive activity. [11]. An example of disease caused by inverse agonists is hypothyroidism. The most common cause of hypothyroidism is Hashimoto’s disease. Without enough TSH to bind TSHR, the pathway remains inactive and thus metabolic processes are inhibited in this pathway. CS-17 interacts with the ECD of the TSHR protein on the convex side GREEN LINK of the LRRD, suppressing TSHR function by keeping the receptor in the inactive state (Figure 3). Clash of bound CS-17 with the cell membrane locks TSHR in the inactive form. This type of inhibition is uncommon and is a promising mechanism for future drug design and research to combat hypothyroidism.[11].
TSH Agonist
This clash is caused by glycosylations of an Asn52 on the . Addition of N-acetyl glucosamine modifications create steric clashes between TSH and the cell membrane, keeping TSHR in the active state.



