We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
===M22 Agonist and Grave's Disease=== | ===M22 Agonist and Grave's Disease=== | ||
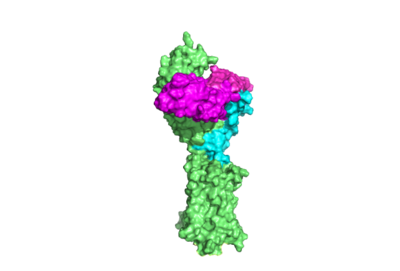
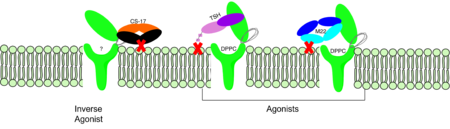
| - | <scene name='95/952708/M22_edited/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that produced by patients with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease effects 1 in 100 Americans and especially women or people older than 30 years of age]. The binding of <scene name='95/952708/Tsh_7t9i/1'>TSH</scene> to <scene name='95/952709/Initial_scene_with_edited_7utz/2'>TSHR</scene> results in the receptor remaining in its active conformation. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR | + | <scene name='95/952708/M22_edited/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that produced by patients with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease effects 1 in 100 Americans and especially women or people older than 30 years of age]. The binding of <scene name='95/952708/Tsh_7t9i/1'>TSH</scene> to <scene name='95/952709/Initial_scene_with_edited_7utz/2'>TSHR</scene> results in the receptor remaining in its active conformation. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR, and is a potent activator for intracellular signaling. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, M22 does not interact with the hinge region when bound to TSHR, whereas TSH bound to TSHR does.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> This finding shows that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 3: Agonist and antagonist drugs for activating or inactivating the TSHR protein. Here the membrane clashes are demonstrated on TSHR with different agonists attached. CS-17 is orange, TSH is purple, and M22 is blue in the figure. The TSHR protein is green and embedded in the protein.]] |
Revision as of 20:43, 12 April 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/
- ↑ 3.0 3.1 3.2 3.3 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 5.0 5.1 5.2 Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 7.0 7.1 7.2 7.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113
- ↑ 10.0 10.1 10.2 10.3 10.4 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 12.0 12.1 Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754