We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1793
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
=== Binding Sites === | === Binding Sites === | ||
==== Sodium ==== | ==== Sodium ==== | ||
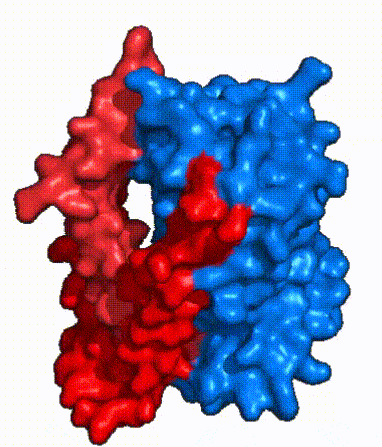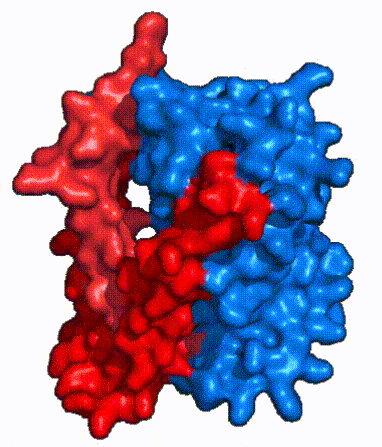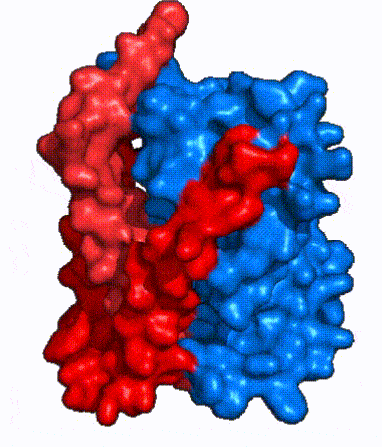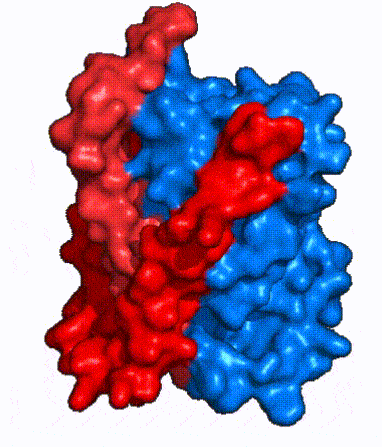
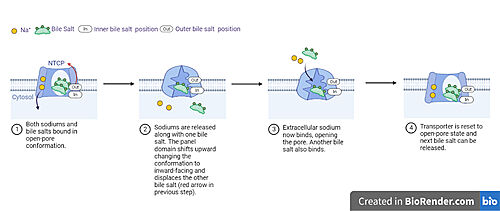
| - | NTCP, like other SLC10 family members, have <scene name='95/952721/Sodium_binding/5'>two sodium binding</scene>. Many polar and negatively charged residues (68, 105, 106, 119, 123, 257, 261) form ion-dipole or dipole-dipole interactions with the sodium ions in these sites with a high level of conservation, suggesting | + | NTCP, like other SLC10 family members, have <scene name='95/952721/Sodium_binding/5'>two sodium binding sites</scene>. Many polar and negatively charged residues (68, 105, 106, 119, 123, 257, 261) form ion-dipole or dipole-dipole interactions with the sodium ions in these sites with a high level of conservation, suggesting sodium binding is coupled to bile salt transport. <Ref name = "Goutam"> Mutations in the X-motif near sodium binding sites inhibit bile salt transport function, suggesting that sodium allows is required for salt binding. |
<Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> Thermodynamically favorable sodium transport facilitates changes in NTCP from open-pore to closed pore states, moving bile salts against their concentration gradient. The inward-facing state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. <Ref name = "Goutam"> This also allows for sodium concentrations to regulate uptake of taurocholates. When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> | <Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> Thermodynamically favorable sodium transport facilitates changes in NTCP from open-pore to closed pore states, moving bile salts against their concentration gradient. The inward-facing state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. <Ref name = "Goutam"> This also allows for sodium concentrations to regulate uptake of taurocholates. When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> | ||
==== Bile Salt ==== | ==== Bile Salt ==== | ||
| - | + | A key feature of NTCP is its <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> which allows for bile salt transport across the hydrophilic membrane. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while the [INSERT GREEN LINK] lining of the open pore is largely {{Template:ColorKey_Polar}}. In the inward-facing or closed-pore conformation, the polar pore residues are inaccessible. Only the surface hydrophobic residues are exposed. As the pore opens up, inner polar residues become accessible allowing for the binding of hydrophilic bile salts. The pattern of hydrophobic and polar residues within the pore matches the amphipathic patterns within taurocholate, [https://en.wikipedia.org/wiki/Steroid steroids], and [https://en.wikipedia.org/wiki/Thyroid_hormones thyroid hormones]. <Ref name = Qi> Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294 </ref> Using this amphipathic pore, provides the channel with specificity while preventing leakage of other substrates. Essential <scene name='95/952722/Bile_salts_res/1'>bile salt binding residues</scene> form Van der Waals interactions with bile salt substrates, while others will form dipole-dipole or ionic interactions. The core domain contributes most of the polar domains, while the panel domain contributes mainly hydrophobic surface. | |
== Conformational Change == | == Conformational Change == | ||
{| | {| | ||
Revision as of 16:38, 14 April 2023
Sodium Bile Salt Co-Transporting Protein
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 4.0 4.1 Mutations in the X-motif near sodium binding sites inhibit bile salt transport function, suggesting that sodium allows is required for salt binding. <ref> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </li> <li id="cite_note-Liu-4">↑ <sup>[[#cite_ref-Liu_4-0|5.0]]</sup> <sup>[[#cite_ref-Liu_4-1|5.1]]</sup> <sup>[[#cite_ref-Liu_4-2|5.2]]</sup> Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4 </li> <li id="cite_note-Latorraca-5">[[#cite_ref-Latorraca_5-0|↑]] Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107. </li> <li id="cite_note-Asami-6">↑ <sup>[[#cite_ref-Asami_6-0|7.0]]</sup> <sup>[[#cite_ref-Asami_6-1|7.1]]</sup> <sup>[[#cite_ref-Asami_6-2|7.2]]</sup> <sup>[[#cite_ref-Asami_6-3|7.3]]</sup> Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al. Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4 </li> <li id="cite_note-Grove-7">[[#cite_ref-Grove_7-0|↑]] Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </li> <li id="cite_note-Herrscher-8">↑ <sup>[[#cite_ref-Herrscher_8-0|9.0]]</sup> <sup>[[#cite_ref-Herrscher_8-1|9.1]]</sup> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </li></ol></ref>