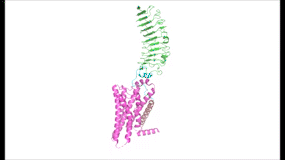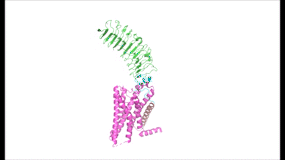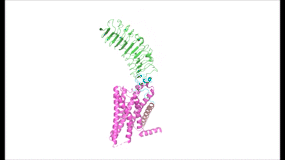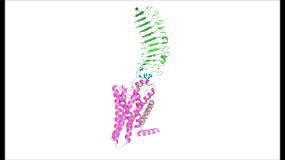We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1774
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Introduction == | == Introduction == | ||
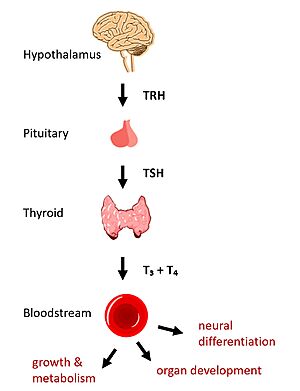
[[Image:HPT Axis.jpg|300 px|right|thumb|Figure 1: TSH binds TSHR on the surface of thyroid cells in the HPT signaling axis pathway, which regulates metabolism and growth.]] | [[Image:HPT Axis.jpg|300 px|right|thumb|Figure 1: TSH binds TSHR on the surface of thyroid cells in the HPT signaling axis pathway, which regulates metabolism and growth.]] | ||
| - | In humans, the hypothalamic-pituitary-thyroid (HPT) signaling axis regulates functions including metabolism, growth, organ development, and neural differentiation <ref name="Brent">Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-3043. [https://doi.org/10.1172/JCI60047 DOI: 10.1172/JCI60047]</ref>. In this pathway, the thyroid stimulating hormone receptor (TSHR) activates transcription of thyroid hormones thyroxine (T3) and triiodothyronine (T4) in response to ligand binding by thyroid stimulating hormone (TSH). After a brief introduction to the biological significance of TSHR, this page explores the structure of TSHR and its significance to TSH binding and receptor activation. | + | In humans, the hypothalamic-pituitary-thyroid (HPT) signaling axis regulates functions including metabolism, growth, organ development, and neural differentiation <ref name="Brent">Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035-3043. [https://doi.org/10.1172/JCI60047 DOI: 10.1172/JCI60047]</ref>. In this pathway, the thyroid stimulating hormone receptor (TSHR) activates transcription of thyroid hormones thyroxine (T3) and triiodothyronine (T4) in response to ligand binding by thyroid stimulating hormone (TSH) <ref name="Chu">Chu YD, Yeh CT. The Molecular Function and Clinical Role of Thyroid Stimulating Hormone Receptor in Cancer Cells. Cells. 2020;9(7):1730. [https://doi.org/10.3390/cells9071730 DOI:10.3390/cells9071730]</ref>. After a brief introduction to the biological significance of TSHR, this page explores the structure of TSHR and its significance to TSH binding and receptor activation. |
== Biological Significance of TSHR == | == Biological Significance of TSHR == | ||
| - | The HPT signaling axis involves the brain, thyroid gland, and bloodstream circulation. In the first step of the pathway, thyrotropin releasing hormone (TRH) is secreted by the hypothalamus, which in turn stimulates the anterior pituitary gland to produce TSH <ref name="Brent" />. TSH binds to TSHR on the surface of thyroid cells and triggers the production of T3 and T4 through [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3967846/ G-protein coupled receptor (GPCR)] signaling <ref name="Chu" | + | The HPT signaling axis involves the brain, thyroid gland, and bloodstream circulation. In the first step of the pathway, thyrotropin releasing hormone (TRH) is secreted by the hypothalamus, which in turn stimulates the anterior pituitary gland to produce TSH <ref name="Brent" />. TSH binds to TSHR on the surface of thyroid cells and triggers the production of T3 and T4 through [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3967846/ G-protein coupled receptor (GPCR)] signaling <ref name="Chu" />. T3 and T4 circulate in the bloodstream and enter cells via thyroid hormone transporters to regulate metabolic functions including neural differentiation, metabolism, and growth and development. Additionally, T3 and T4 act in a negative feedback loop to inhibit further TSH production <ref name="Brent" />. |
Dysregulation of TSHR can lead to disease. In [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease#:~:text=Graves'%20disease%20is%20an%20autoimmune,the%20way%20your%20heart%20beats. Grave's disease], antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism <ref name="Chu" />. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated <ref name="Brent" />. | Dysregulation of TSHR can lead to disease. In [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease#:~:text=Graves'%20disease%20is%20an%20autoimmune,the%20way%20your%20heart%20beats. Grave's disease], antibody analogs of TSH cause overactivation of TSHR, leading to clinical symptoms of hyperthyroidism <ref name="Chu" />. In contrast, congenital mutations which inactivate TSHR can lead to hypothyroidism, which results in growth retardation and neurologic impairment if left untreated <ref name="Brent" />. | ||
| Line 15: | Line 15: | ||
== Molecular Structure and Function of TSHR == | == Molecular Structure and Function of TSHR == | ||
=== Role of TSHR Domains === | === Role of TSHR Domains === | ||
| - | To understand how the structure of TSHR contributes to its function, it is helpful to | + | To understand how the structure of TSHR contributes to its function, it is helpful to become familiar with the three main domains of TSHR. First is the <scene name='95/952703/Tmd/9'>extracellular domain (ECD)</scene>, which is concave in shape. It is also called the leucine rich region because it is made primarily of beta sheets which are rich in leucine <ref name="Kleinau">Kleinau G, Worth CL, Kreuchwig A, et al. Structural–Functional Features of the Thyrotropin Receptor: A Class A G-Protein-Coupled Receptor at Work. Frontiers in Endocrinology. 2017;8. Accessed April 2, 2023. [https://doi.org/10.3389/fendo.2017.00086 DOI: 10.3389/fendo.2017.00086]</ref>. The ECD contains lysine residues which play a key role in TSH binding. Second is the <scene name='95/952703/Tmd/10'>transmembrane domain (TMD) </scene>, which is composed of seven transmembrane alpha helices which are connected by extracellular loops (ECL). The TMD undergoes a conformation change upon ligand binding that activates the intracellular GPCR signal cascade <ref name="Duan" />. The third region of the TSHR is the <scene name='95/952703/Tmd/11'>hinge region</scene>, which plays a key role in the movement and stability of the TSHR. The details of the hinge mechanism are discussed in the proceeding section. |
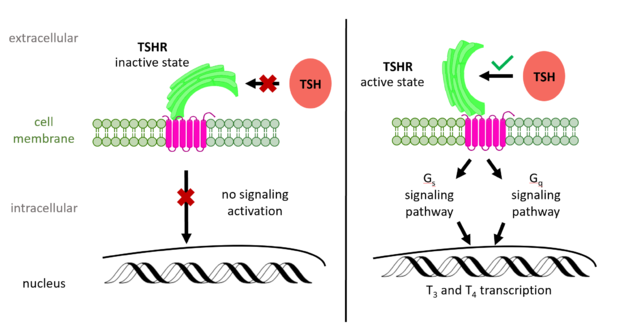
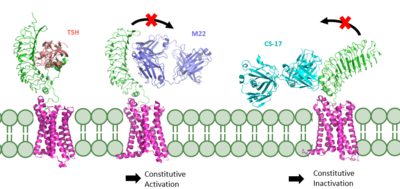
| + | [[Image:TSH Signaling.png|right|620 px|thumb|Figure 2: (Left) In the downright, inactive state, TSH cannot bind and no signaling activation occurs. (Right) In the upright, active state, binding of TSH leads to GPCR signaling activation and production of T3 and T4 hormones.]] | ||
=== Importance of Hinge Region to Signaling Activation === | === Importance of Hinge Region to Signaling Activation === | ||
| - | + | The TSHR hinges between two states: <scene name='95/952702/Overlay/2'>active and inactive</scene>. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. When the extracellular domain raises into the upright position, the hinge region deforms and interacts with the extracellular loops to cause a conformation change in the TMD and corresponding G-protein activation <ref name="Bruser">Bruser A, Schulz A, Rothemund S, et al. The Activation Mechanism of Glycoprotein Hormone Receptors with Implications in the Cause and Therapy of Endocrine Diseases. J Biol Chem. 2016;291(2):508-520. [https://doi.org/10.1074/jbc.M115.701102 DOI:10.1074/jbc.M115.701102]</ref>. While transition between the active and inactive states occurs spontaneously, favoring of one state over the other is influence by hinge interactions and ligand binding <ref name="Faust">PMID:35940205</ref>. When stabilized in the upright conformation, the activated GPCR signaling pathway results in transcription of thyroid hormones T3 and T4 (Fig. 2) <ref name="Chu" />. | |
| - | + | ||
| - | The TSHR hinges between two states: <scene name='95/952702/Overlay/2'>active and inactive</scene>. When the extracellular domain is hinged down, the receptor is inactive and no signaling activation occurs. When the extracellular domain raises into the upright position, the hinge region deforms and interacts with the extracellular loops to cause a conformation change in the | + | |
Modulation of TSHR signaling would not be possible without the hinge region, which accommodates up-and-down rotation of the extracellular domain as a rigid body about an imaginary 55 degree axis <ref name="Faust" />. During this transition, the hinge region undergoes <scene name='95/952702/P10_movement/4'>slinky-like deformation</scene> and is displaced approximately 5 Angstroms upward as it uncoils <ref name="Faust" />. The hinge region pulls on the linked transmembrane helices as it stretches, shifting <scene name='95/952702/Helix7_movement/2'>TM helix 7</scene> approximately 4 Angstroms inward and leading to G-protein signaling activation <ref name="Faust" />, <ref name="Bruser" />. | Modulation of TSHR signaling would not be possible without the hinge region, which accommodates up-and-down rotation of the extracellular domain as a rigid body about an imaginary 55 degree axis <ref name="Faust" />. During this transition, the hinge region undergoes <scene name='95/952702/P10_movement/4'>slinky-like deformation</scene> and is displaced approximately 5 Angstroms upward as it uncoils <ref name="Faust" />. The hinge region pulls on the linked transmembrane helices as it stretches, shifting <scene name='95/952702/Helix7_movement/2'>TM helix 7</scene> approximately 4 Angstroms inward and leading to G-protein signaling activation <ref name="Faust" />, <ref name="Bruser" />. | ||
Revision as of 15:35, 15 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||