We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
| + | === Active and Inactive Form === | ||
=== Active and Inactive Form === | === Active and Inactive Form === | ||
[[Image:Finalmorphpic2.png|450 px|right|thumb|Figure 2: Inactive form of the thyrotropin receptor shown in blue (PDB: 7T9M). Active form of the thyrotropin receptor shown in green (PDB: 7T9I).]] | [[Image:Finalmorphpic2.png|450 px|right|thumb|Figure 2: Inactive form of the thyrotropin receptor shown in blue (PDB: 7T9M). Active form of the thyrotropin receptor shown in green (PDB: 7T9I).]] | ||
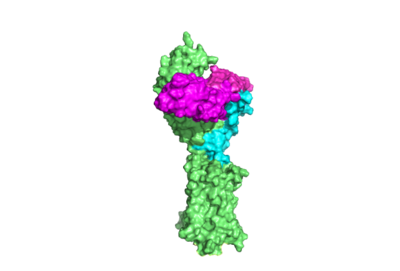
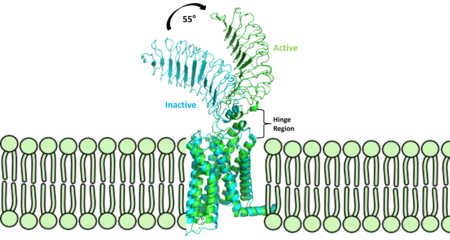
| - | The TSHR protein exists in dynamic equilibrium between two states: active and inactive (Figure 2 | + | The TSHR protein exists in dynamic equilibrium between two states: active and inactive (Figure 2). <scene name='95/952708/Tsh_7t9i/2'>TSH</scene> will bind and keep the active state in the up position as a result of clashes between bound TSH and the cell membrane.<ref name="Faust" />. <scene name='95/952708/Tsh_7t9i/4'>Glycolysations of an ASN52 residue</scene> cause this clash on the <scene name='95/952707/Tsh_7t9i/1'>α-subunit of TSH</scene>. |
===Structural Overview=== | ===Structural Overview=== | ||
The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the ECD to the intracellular loops <ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. Thyrotropin binding causes a conformational change in the ECD that is transduced through the transmembrane helices. In the active state, the ECD is in the "up" position, while in the inactive state, the ECD is in the "down" state, closer to the cell membrane. A "push-pull" mechanism is proposed for the ECD's conformational change between active and inactive states. In the "push" model, TSH binds to the receptor and sterically clashes with the cellular membrane, forcing the ECD up away from the membrane. In the pull model, a short α-helix interacts with TSH to pull the ECD up. The active (up) form of the ECD causes a conformation shift in the TMD which causes differential interactions with a heterotrimeric <scene name='95/952709/G_protein/2'>G-protein</scene>, initiating intracellular signaling<ref name="Duan et al.">PMID:35940204</ref>. | The thyrotropin receptor has an extracellular domain (ECD) that is composed of a <scene name='95/952709/Lrrd_real/2'>leucine rich repeat domain (LRRD)</scene> as well as a hinge region. The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> links the ECD to the seven transmembrane helices <scene name='95/952709/7tm_helices/4'>(7TM domain)</scene>, which span from the ECD to the intracellular loops <ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. Thyrotropin binding causes a conformational change in the ECD that is transduced through the transmembrane helices. In the active state, the ECD is in the "up" position, while in the inactive state, the ECD is in the "down" state, closer to the cell membrane. A "push-pull" mechanism is proposed for the ECD's conformational change between active and inactive states. In the "push" model, TSH binds to the receptor and sterically clashes with the cellular membrane, forcing the ECD up away from the membrane. In the pull model, a short α-helix interacts with TSH to pull the ECD up. The active (up) form of the ECD causes a conformation shift in the TMD which causes differential interactions with a heterotrimeric <scene name='95/952709/G_protein/2'>G-protein</scene>, initiating intracellular signaling<ref name="Duan et al.">PMID:35940204</ref>. | ||
| Line 17: | Line 18: | ||
The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the LRRD to the 7TMD. The hinge region also impacts TSH binding potency and intracellular cyclic adenosine monophosphate (cAMP) levels, mediated by the activation of the GPCR<ref name="Mizutori et al.">Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407</ref>. The hinge region's <scene name='95/952709/Hinge_helix_rotation/1'>hinge helix</scene> interacts with the <scene name='95/952709/P10_peptide_region/2'>p10 peptide</scene> through disulfides. The p10 peptide is a conserved sequence that spans from the last β sheet of the LRRD to the first transmembrane helix (TM1) and is an intramolecular agonist for conformational shifts in the 7TMD helices<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. The disulfides between the LRRD, the hinge helix, and the p10 are critical to TSH signaling as they transduce signal from the ECD through the hinge helix to the p10 peptide. The upward movement of the LRRD, caused by TSH binding, will cause rotation of the hinge helix. The subsequent movement of the p10 peptide leads to movement of the transmembrane helices, which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing two key residues. Y385 from TSHR is buried into a hydrophobic pocket of TSH. D386 from the receptor forms a salt bridge with R386 of the hormone. <scene name='95/952709/Binding_interactions_hinge/1'>These interactions</scene> that assist in the stable binding of TSH to TSHR allow more potent activation of the receptor<ref name="Duan et al.">PMID:35940204</ref>. Even with these key functions, the hinge region itself is not absolutely required for receptor activation<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. The hinge region functions as a point of attachment to the 7TMD for the LRRD, and its ability to rotate allows for LRRD shifts between up (active state) and down (inactive state) positions. The interactions that the hinge helix makes with the LRRD and p10 act as an important communication medium between the ECD and an intramolecular agonist directly effecting conformational shifts in the 7TMD. | The <scene name='95/952709/Hinge_region_real/2'>hinge region</scene> is a scaffold for the attachment of the LRRD to the 7TMD. The hinge region also impacts TSH binding potency and intracellular cyclic adenosine monophosphate (cAMP) levels, mediated by the activation of the GPCR<ref name="Mizutori et al.">Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407</ref>. The hinge region's <scene name='95/952709/Hinge_helix_rotation/1'>hinge helix</scene> interacts with the <scene name='95/952709/P10_peptide_region/2'>p10 peptide</scene> through disulfides. The p10 peptide is a conserved sequence that spans from the last β sheet of the LRRD to the first transmembrane helix (TM1) and is an intramolecular agonist for conformational shifts in the 7TMD helices<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. The disulfides between the LRRD, the hinge helix, and the p10 are critical to TSH signaling as they transduce signal from the ECD through the hinge helix to the p10 peptide. The upward movement of the LRRD, caused by TSH binding, will cause rotation of the hinge helix. The subsequent movement of the p10 peptide leads to movement of the transmembrane helices, which will cause activation of the G-protein. In addition to activation, the hinge region plays an important role in tightly binding TSH. Residues 382-390 of the hinge region adopt a short helix containing two key residues. Y385 from TSHR is buried into a hydrophobic pocket of TSH. D386 from the receptor forms a salt bridge with R386 of the hormone. <scene name='95/952709/Binding_interactions_hinge/1'>These interactions</scene> that assist in the stable binding of TSH to TSHR allow more potent activation of the receptor<ref name="Duan et al.">PMID:35940204</ref>. Even with these key functions, the hinge region itself is not absolutely required for receptor activation<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref>. The hinge region functions as a point of attachment to the 7TMD for the LRRD, and its ability to rotate allows for LRRD shifts between up (active state) and down (inactive state) positions. The interactions that the hinge helix makes with the LRRD and p10 act as an important communication medium between the ECD and an intramolecular agonist directly effecting conformational shifts in the 7TMD. | ||
===7 Transmembrane Helices=== | ===7 Transmembrane Helices=== | ||
| - | The ECD of TSHR is anchored to the membrane through seven transmembrane helices (7TMD), characteristic of GPCRs. Conformational changes in the 7TMD activate intracellular G-protein signaling<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. Once TSH binds, conformational changes to the p10 peptide are transmitted to the 7TMD. Specifically, hinge helix rotation causes the displacement of the p10 peptide that allows the <scene name='95/952709/Helix_7_of_7tmd/2'>seventh transmembrane helix (TM7)</scene> to migrate towards the center of the 7TMD, increasing van Der Waals contacts. Additionally, K660 of TM7 forms a stabilizing <scene name='95/952709/Helix_7_and__p10_interaction/6'>ionic interaction</scene> with E409 of the p10 region. Hinge helix movement also rearranges Y279 relative to I486 on the neighboring <scene name='95/952709/Hinge_helix_ecl1/3'>extracellular loop 1 (ECL1) helix</scene>, which links two transmembrane helices and is located extracellularly. Substitution of these residues leads to substantial shifts in the activation of the thyrotropin receptor. Structurally guided mutagenic studies have shown that replacing isoleucine with a more sizeable phenylalanine decreases TSH signaling potency<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Vlaeminck-Guillem et al.">Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816</ref>.The sixth transmembrane helix of TSHR moves outward from the center of the 7TMD to facilitate α-helix 5 of the α-subunit of the G protein (Gα)<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Goricanec et al.">Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113 </ref>. Gα is activated for intracellular signaling when GDP is exchanged for GTP and dissociates from the γ- and β-subunits of the G-protein (Gγ and Gβ) to bind with other target proteins. Activation of the Gα is caused by conformational shifts in the 7TMD and three intracellular loops which directly interact with the G-protein<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. These conformational shifts in transmembrane helices are the mechanism of changing interactions of the G-protein with the receptor. | + | The ECD of TSHR is anchored to the membrane through seven transmembrane helices (7TMD), characteristic of GPCRs. Conformational changes in the 7TMD activate intracellular G-protein signaling<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. Once TSH binds, conformational changes to the p10 peptide are transmitted to the 7TMD. Specifically, hinge helix rotation causes the displacement of the p10 peptide that allows the <scene name='95/952709/Helix_7_of_7tmd/2'>seventh transmembrane helix (TM7)</scene> to migrate towards the center of the 7TMD, increasing van Der Waals contacts. Additionally, K660 of TM7 forms a stabilizing <scene name='95/952709/Helix_7_and__p10_interaction/6'>ionic interaction</scene> with E409 of the p10 region. Hinge helix movement also rearranges Y279 relative to I486 on the neighboring <scene name='95/952709/Hinge_helix_ecl1/3'>extracellular loop 1 (ECL1) helix</scene>, which links two transmembrane helices and is located extracellularly. Substitution of these residues leads to substantial shifts in the activation of the thyrotropin receptor. Structurally guided mutagenic studies have shown that replacing isoleucine with a more sizeable phenylalanine decreases TSH signaling potency<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Vlaeminck-Guillem et al.">Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816</ref>.The sixth transmembrane helix of TSHR moves outward from the center of the 7TMD to facilitate α-helix 5 of the α-subunit of the G protein (Gα)<ref name="Faust et al.">Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-</ref><ref name="Goricanec et al.">Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113 </ref>. Gα is activated for intracellular signaling when GDP is exchanged for GTP and dissociates from the γ- and β-subunits of the G-protein (Gγ and Gβ) to bind with other target proteins. Activation of the Gα is caused by conformational shifts in the 7TMD and three intracellular loops which directly interact with the G-protein<ref name= "Keinau et al.">Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086</ref>. These conformational shifts in transmembrane helices are the mechanism of changing interactions of the G-protein with the receptor. |
| - | + | ||
| - | + | ||
== TSHR Agonists and Antagonists == | == TSHR Agonists and Antagonists == | ||
| Line 26: | Line 25: | ||
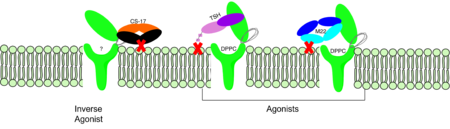
<scene name='95/952708/M22_edited/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that is produced by patients with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease effects 1 in 100 Americans and especially women or people older than 30 years of age]. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and the cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR, and is a potent activator for intracellular signaling. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, M22 does not interact with the hinge region when bound to TSHR.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> These findings show that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 3: Agonist and antagonist drugs for activating or inactivating the TSHR protein. Here the membrane clashes are demonstrated on TSHR with different agonists attached. CS-17 is orange, TSH is purple, and M22 is blue in the figure. The TSHR protein is green and embedded in the protein.]] | <scene name='95/952708/M22_edited/3'>M22</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that is produced by patients with [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease Graves' Disease]. In Graves' disease, autoantibodies mimic TSH function and cause thyroid overactivity. <ref name="Miguel"> doi:10.1677/JME-08-0152</ref>. Grave's Disease is an autoimmune disease that is a result of hyperthyroidism, where too much TSH is being produced. This disease [https://www.niddk.nih.gov/health-information/endocrine-diseases/graves-disease effects 1 in 100 Americans and especially women or people older than 30 years of age]. The M22 [https://en.wikipedia.org/wiki/Autoantibody autoantibody] activates TSHR by causing a membrane clash with the ECD and the cell membrane, keeping the TSHR in the active state by preventing the TSHR from rotating to the inactive state (Figure 3). M22 mimics TSH activation of TSHR, and is a potent activator for intracellular signaling. <ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> Although M22 binds in a similar manner to TSH, M22 does not interact with the hinge region when bound to TSHR.<ref name="Faust"> DOI:10.1038/s41586-022-05159-1</ref> These findings show that the hinge region is not necessary for the activation of TSHR, and leads to the discovery of other methods of activation. [[Image:Agonist pic.png|450 px|right|thumb|Figure 3: Agonist and antagonist drugs for activating or inactivating the TSHR protein. Here the membrane clashes are demonstrated on TSHR with different agonists attached. CS-17 is orange, TSH is purple, and M22 is blue in the figure. The TSHR protein is green and embedded in the protein.]] | ||
===CS-17 Inverse Agonist=== | ===CS-17 Inverse Agonist=== | ||
| - | <scene name='95/ | + | <scene name='95/952708/Cs17/1'>CS-17</scene> is a [https://en.wikipedia.org/wiki/Monoclonal_antibody monoclonal antibody] that acts as an inverse agonist for TSHR constitutive activity. <ref name= "Chen et al.">Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754</ref>. An example of a disease caused by inverse agonists is [https://www.mayoclinic.org/diseasesconditions/hypothyroidism/symptomscauses/syc20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages hypothyroidism]. The most common cause of hypothyroidism is [https://www.mayoclinic.org/diseasesconditions/hypothyroidism/symptomscauses/syc20350284#:~:text=Hypothyroidism%20happens%20when%20the%20thyroid,symptoms%20in%20its%20early%20stages Hashimoto’s disease]. Without enough TSH to bind TSHR, the pathway remains inactive and thus metabolic processes are inhibited in this pathway. CS-17 interacts with the ECD of the TSHR protein on the |
| + | <scene name='95/952708/Cs17/2'>convex side</scene> of the LRRD, suppressing TSHR function by keeping the receptor in the inactive state (Figure 3). Clash of bound CS-17 with the cell membrane locks TSHR in the inactive form. This type of inhibition is uncommon and is a promising mechanism for future drug design and research to combat hypothyroidism.<ref name="Chen et al.">Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754</ref>. | ||
Revision as of 20:16, 15 April 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/
- ↑ 3.0 3.1 3.2 3.3 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 5.0 5.1 5.2 5.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 6.0 6.1 6.2 Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 8.0 8.1 8.2 8.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113
- ↑ 11.0 11.1 Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 12.0 12.1 Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754