We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1783
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
== Structural Overview == | == Structural Overview == | ||
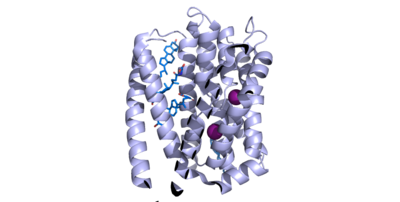
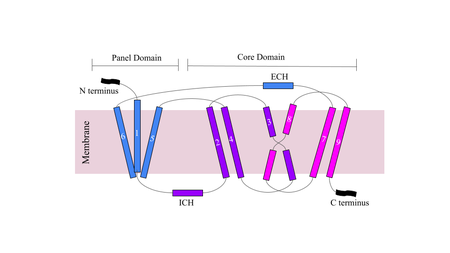
| - | NTCP has 9 transmembrane alpha helices (TM) that form the protein, with an extracellular N-terminus and intracellular C-terminus. NTCP has two domains within the protein, <scene name='95/952711/Panel_domain/ | + | NTCP has 9 transmembrane alpha helices (TM) that form the protein, with an extracellular N-terminus and intracellular C-terminus. NTCP has two domains within the protein, <scene name='95/952711/Panel_domain/3'>a panel domain</scene>, made up of TM1, TM5, and TM6, and <scene name='95/952711/Core_domain/4'>a core domain</scene>, made up of TM2-4 and TM7-9. An interesting feature of NTCP is the cross of TM3 and TM8 that form an <scene name='95/952711/X_motif/2'>an X motif</scene> within the protein that is used in the conformational change of NTCP. The two domains are essential to the conformation change of NTCP to bind bile salts. The intracellular α-helix (ICH) connects TM1 and TM2. The extracellular α-helix connects TM6 and TM7. <Ref name="Xiangbing"> Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, [https://doi.org/10.1016/j.xinn.2022.100294 DOI: 10.1016/j.xinn.2022.100294]. </Ref> |
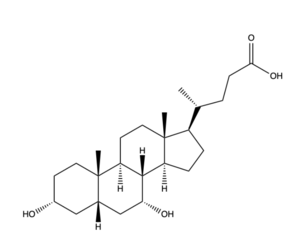
There are two significant patches in the NTCP structure that facilitate ligand binding. Residues 84-87 of NTCP are <scene name='95/952711/Binding_patch_1/2'>in Patch 1</scene>, which is located on the TM2-TM3 loop in the core domain. This patch is also considered the extracellular region of the binding tunnel within NTCP. Residues 157-165 of NTCP are associated with <scene name='95/952711/Binding_site_2_with_surface/2'>Patch 2</scene>. They are located on the N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in the extracellular region of the binding tunnel. These residues' importance was determined through mutations of these residues and examined through pull-down assays. <ref name="Asami"/> The pre-S1 domain of HBV/HDV binds to the patches on NTCP in order to transport the virus from the exterior of NTCP to the interior binding tunnel to infect human liver cells.<ref name="Goutam"/> | There are two significant patches in the NTCP structure that facilitate ligand binding. Residues 84-87 of NTCP are <scene name='95/952711/Binding_patch_1/2'>in Patch 1</scene>, which is located on the TM2-TM3 loop in the core domain. This patch is also considered the extracellular region of the binding tunnel within NTCP. Residues 157-165 of NTCP are associated with <scene name='95/952711/Binding_site_2_with_surface/2'>Patch 2</scene>. They are located on the N-terminal half of the TM5 in the panel domain (residue sequence: KGIVISLVL). Patch 2 is also located in the extracellular region of the binding tunnel. These residues' importance was determined through mutations of these residues and examined through pull-down assays. <ref name="Asami"/> The pre-S1 domain of HBV/HDV binds to the patches on NTCP in order to transport the virus from the exterior of NTCP to the interior binding tunnel to infect human liver cells.<ref name="Goutam"/> | ||
Revision as of 23:42, 16 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
Sodium Taurocholate Co-Transporting Peptide
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ Maldonado-Valderrama, J., Wilde, P., Macierzanka, A., & Mackie, A. (2011). The role of bile salts in digestion. Advances in colloid and interface science, 165(1), 36–46. DOI: 10.1016/j.cis.2010.12.002.
- ↑ 3.0 3.1 Asami J, Kimura KT, Fujita-Fujiharu Y, Ishida H, Zhang Z, Nomura Y, Liu K, Uemura T, Sato Y, Ono M, Yamamoto M, Noda T, Shigematsu H, Drew D, Iwata S, Shimizu T, Nomura N, Ohto U. Structure of the bile acid transporter and HBV receptor NTCP. Nature. 2022 Jun; 606 (7916):1021-1026. DOI: 10.1038/s41586-022-04845-4.
- ↑ Xiangbing Qi, Wenhui Li. (2022). Unlocking the secrets to human NTCP structure. The Innovation, Vol. 3, Issue 5. 100294, ISSN 2666-6758, DOI: 10.1016/j.xinn.2022.100294.
- ↑ Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. The structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). DOI: 10.1038/s41422-022-00680-4.
- ↑ Vlahcevic, Z., Buhac, I., et al. Bile Acid Metabolism in Patients with Cirrhosis. Gastroenterology vol. 60, 491-498 (1971). DOI: 10.1016/S0016-5085(71)80053-7.