We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1779
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
=== Active and Inactive Form === | === Active and Inactive Form === | ||
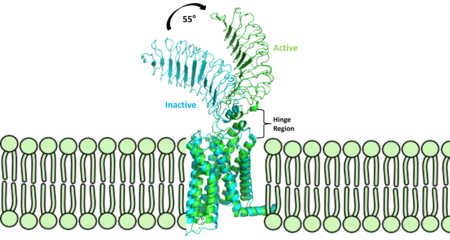
| - | [[Image:Finalmorphpic2.png|450 px|right|thumb|Figure 2: Inactive form of the thyrotropin receptor shown in blue (PDB: 7T9M). Active form of the thyrotropin receptor shown in green (PDB: 7T9I).]] | + | [[Image:Finalmorphpic2.png|450 px|right|thumb|Figure 2: Inactive form of the thyrotropin receptor shown in blue (PDB:[https://www.rcsb.org/structure/7T9M 7T9M]). Active form of the thyrotropin receptor shown in green (PDB:[https://www.rcsb.org/structure/7T9I 7T9I]).]] |
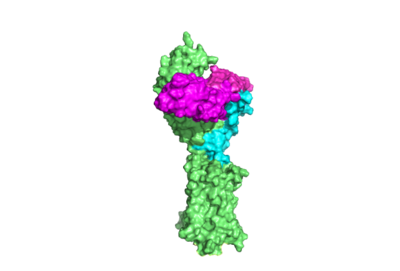
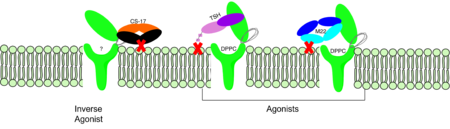
The TSHR protein exists in dynamic equilibrium between two states: active and inactive (Figure 2). <scene name='95/952708/Tsh_7t9i/2'>TSH</scene> will bind and keep the active state in the up position as a result of clashes between bound TSH and the cell membrane.<ref name="Faust" />. <scene name='95/952708/Tsh_7t9i/4'>Glycolysations of an ASN52 residue</scene> cause this clash on the <scene name='95/952707/Tsh_7t9i/1'>α-subunit of TSH</scene>. | The TSHR protein exists in dynamic equilibrium between two states: active and inactive (Figure 2). <scene name='95/952708/Tsh_7t9i/2'>TSH</scene> will bind and keep the active state in the up position as a result of clashes between bound TSH and the cell membrane.<ref name="Faust" />. <scene name='95/952708/Tsh_7t9i/4'>Glycolysations of an ASN52 residue</scene> cause this clash on the <scene name='95/952707/Tsh_7t9i/1'>α-subunit of TSH</scene>. | ||
===Structural Overview=== | ===Structural Overview=== | ||
Revision as of 01:09, 17 April 2023
>
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Yen PM. Physiological and molecular basis of thyroid hormone action. Physiol Rev. 2001 Jul;81(3):1097-142. doi: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
- ↑ Pirahanchi Y, Toro F, Jialal I. Physiology, Thyroid Stimulating Hormone. [Updated 2022 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499850/
- ↑ 3.0 3.1 3.2 3.3 Duan J, Xu P, Luan X, Ji Y, He X, Song N, Yuan Q, Jin Y, Cheng X, Jiang H, Zheng J, Zhang S, Jiang Y, Xu HE. Hormone- and antibody-mediated activation of the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05173-3. doi:, 10.1038/s41586-022-05173-3. PMID:35940204 doi:http://dx.doi.org/10.1038/s41586-022-05173-3
- ↑ Kohn LD, Shimura H, Shimura Y, Hidaka A, Giuliani C, Napolitano G, Ohmori M, Laglia G, Saji M. The thyrotropin receptor. Vitam Horm. 1995;50:287-384. doi: 10.1016/s0083-6729(08)60658-5. PMID: 7709602.
- ↑ 5.0 5.1 5.2 5.3 Faust B, Billesbolle CB, Suomivuori CM, Singh I, Zhang K, Hoppe N, Pinto AFM, Diedrich JK, Muftuoglu Y, Szkudlinski MW, Saghatelian A, Dror RO, Cheng Y, Manglik A. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature. 2022 Aug 8. pii: 10.1038/s41586-022-05159-1. doi:, 10.1038/s41586-022-05159-1. PMID:35940205 doi:http://dx.doi.org/10.1038/s41586-022-05159-1
- ↑ 6.0 6.1 6.2 Kleinau, G., Worth, C. L., Kreuchwig, A., Biebermann, H., Marcinkowski, P., Scheerer, P., & Krause, G. (2017). Structural–functional features of the thyrotropin receptor: A class A G-protein-coupled receptor at work. Frontiers in Endocrinology, 8. https://doi.org/10.3389/fendo.2017.00086
- ↑ Yumiko Mizutori, Chun-Rong Chen, Sandra M. McLachlan, Basil Rapoport, The Thyrotropin Receptor Hinge Region Is Not Simply a Scaffold for the Leucine-Rich Domain but Contributes to Ligand Binding and Signal Transduction, Molecular Endocrinology, Volume 22, Issue 5, 1 May 2008, Pages 1171–1182, https://doi.org/10.1210/me.2007-0407
- ↑ 8.0 8.1 8.2 8.3 Faust, B., Billesbølle, C.B., Suomivuori, CM. et al. Autoantibody mimicry of hormone action at the thyrotropin receptor. Nature 609, 846–853 (2022). https://doi.org/10.1038/s41586-022-
- ↑ Virginie Vlaeminck-Guillem, Su-Chin Ho, Patrice Rodien, Gilbert Vassart, Sabine Costagliola, Activation of the cAMP Pathway by the TSH Receptor Involves Switching of the Ectodomain from a Tethered Inverse Agonist to an Agonist, Molecular Endocrinology, Volume 16, Issue 4, 1 April 2002, Pages 736–746, https://doi.org/10.1210/mend.16.4.0816
- ↑ Goricanec, D., Stehle, R., Egloff, P., Grigoriu, S., Plückthun, A., Wagner, G., & Hagn, F. (2016). Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proceedings of the National Academy of Sciences, 113(26). https://doi.org/10.1073/pnas.1604125113
- ↑ 11.0 11.1 Nunez Miguel R, Sanders J, Chirgadze DY, Furmaniak J, Rees Smith B. Thyroid stimulating autoantibody M22 mimics TSH binding to the TSH receptor leucine rich domain: a comparative structural study of protein-protein interactions. J Mol Endocrinol. 2009 May;42(5):381-95. Epub 2009 Feb 16. PMID:19221175 doi:10.1677/JME-08-0152
- ↑ 12.0 12.1 Chen, C.-R., McLachlan, S. M., & Rapoport, B. (2007). Suppression of thyrotropin receptor constitutive activity by a monoclonal antibody with inverse agonist activity. Endocrinology, 148(5), 2375–2382. https://doi.org/10.1210/en.2006-1754