We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1777
From Proteopedia
(Difference between revisions)
| Line 29: | Line 29: | ||
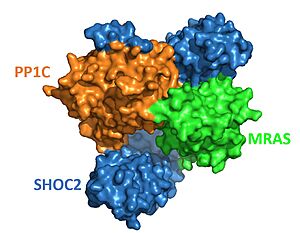
A key feature in allowing MRAS to be in its active versus inactive state is the switches in MRAS. When MRAS is active, GTP is bound and the switches allow MRAS to be bound with PP1C. When MRAS is inactive, GDP is bound and the switches do not allow MRAS to bind to PP1C. The switches determine whether MRAS can bind to SHOC2-PP1C. The switches have to go through a conformational change to allow binding of SHOC2-PP1C to MRAS. The conformational change is needed because without it SHOC2-PP1C could bind to MRAS when MRAS is still inactive. This process would cause the SHOC2-PP1C-MRAS pathway to constantly be running. The switches and GDP/GTP help regulate this process. | A key feature in allowing MRAS to be in its active versus inactive state is the switches in MRAS. When MRAS is active, GTP is bound and the switches allow MRAS to be bound with PP1C. When MRAS is inactive, GDP is bound and the switches do not allow MRAS to bind to PP1C. The switches determine whether MRAS can bind to SHOC2-PP1C. The switches have to go through a conformational change to allow binding of SHOC2-PP1C to MRAS. The conformational change is needed because without it SHOC2-PP1C could bind to MRAS when MRAS is still inactive. This process would cause the SHOC2-PP1C-MRAS pathway to constantly be running. The switches and GDP/GTP help regulate this process. | ||
| - | This conformational change is caused by GTP replacing GDP. When GTP is bound, MRAS shifts and binds with the previously associated SHOC2-PP1C complex. When GDP is bound, <scene name='95/952705/Switch_i_and_ii_with_gdp/8'> | + | This conformational change is caused by GTP replacing GDP. When GTP is bound, MRAS shifts and binds with the previously associated SHOC2-PP1C complex. When GDP is bound, <scene name='95/952705/Switch_i_and_ii_with_gdp/8'>switch II</scene> in MRAS is moved outward, which causes a steric clash with SHOC2 <scene name='95/952705/Gdp_with_mras_and_shoc2/2'>(SHOC2 with Closed Switches)</scene>. When GTP is bound, <scene name='95/952705/Switch_i_and_ii_with_gtp/5'>Switch II</scene> in MRAS can form various hydrogen bonding, pi stacking and hydrophobic interactions with SHOC2<Ref name='Bonsor'>Daniel A. Bonsor, Patrick Alexander, Kelly Snead, Nicole Hartig, Matthew Drew, Simon Messing, Lorenzo I. Finci, Dwight V. Nissley, Frank McCormick, Dominic Esposito, Pablo Rodrigiguez-Viciana, Andrew G. Stephen, Dhirendra K. Simanshu. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. bioRxiv. 2022.05.10.491335. doi: 10.1101/2022.05.10.491335. [https://doi.org/10.1101/2022.05.10.491335. DOI:10.1101/2022.05.10.491335]. </Ref>. When MRAS is bound to SHOC2-PP1C, switch I has an important role in making interactions with PP1C<scene name='95/952705/Switch_i_and_ii_with_gtp/7'>(SHOC2 with Open Switches)</scene>. Though it is minute there is a change of the positioning of switch II when GDP vs GTP is bound. |
==Stabilizing Interactions in Ternary Complex== | ==Stabilizing Interactions in Ternary Complex== | ||
| Line 41: | Line 41: | ||
Notably, NTpS can bind to the PP1C active site without PP1C being in complex with SHOC2 and MRAS. This occurs when RAS binds to RAF, which exposes NTpS on RAF. However, for this reaction, the catalytic activity of PP1C outside of the complex is less efficient. It's hypothesized this is because hydrophobic residues directly C-terminal to the phosphorylated serine bind to the hydrophobic patch of PP1C as well as the hydrophobic SHOC2 C-terminus<ref name="Liau">PMID:35768504</ref>. | Notably, NTpS can bind to the PP1C active site without PP1C being in complex with SHOC2 and MRAS. This occurs when RAS binds to RAF, which exposes NTpS on RAF. However, for this reaction, the catalytic activity of PP1C outside of the complex is less efficient. It's hypothesized this is because hydrophobic residues directly C-terminal to the phosphorylated serine bind to the hydrophobic patch of PP1C as well as the hydrophobic SHOC2 C-terminus<ref name="Liau">PMID:35768504</ref>. | ||
| - | The conserved hydrophobic groove at the SHOC2 C-terminus and the hydrophobic region next to the PP1C active site neighbor each other in the structure of the complex. As a result, the hydrophobic residues downstream from NTpS bind to the hydrophobic patch of PP1C next to the active site and to the hydrophobic groove in the SHOC2 C-terminus | + | The conserved hydrophobic groove at the SHOC2 C-terminus and the hydrophobic region next to the PP1C active site neighbor each other in the structure of the complex. As a result, the hydrophobic residues downstream from NTpS bind to the hydrophobic patch of PP1C next to the active site and to the hydrophobic groove in the SHOC2 C-terminus. There is still some uncertainty as to how the substrate selectivity works, but these regions could play an essential role in the promiscuous binding of PP1C.<ref name="Liau">PMID:35768504</ref>. |
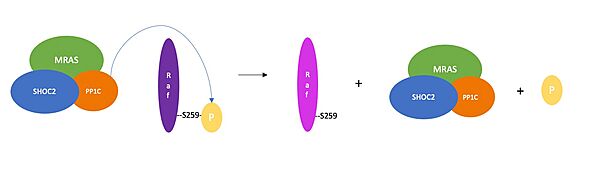
=Activation of RAF= | =Activation of RAF= | ||
Revision as of 16:51, 17 April 2023
| This Sandbox is Reserved from February 27 through August 31, 2023 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1765 through Sandbox Reserved 1795. |
To get started:
More help: Help:Editing |
| |||||||||||
References
- ↑ 1.0 1.1 Bernal Astrain G, Nikolova M, Smith MJ. Functional diversity in the RAS subfamily of small GTPases. Biochem Soc Trans. 2022 Apr 29;50(2):921-933. doi: 10.1042/BST20211166. DOI:10.1042/BST20211166
- ↑ 2.0 2.1 Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006 Jan;1(1):7-9. DOI:10.1016/S1556-0864(15)31506-9.
- ↑ Li, L., Zhao, G. D., Shi, Z. et. al.The Ras/Raf/MEK/ERK signaling pathway (Figure 1) and its role in the occurrence and development of HCC. Oncology letters, 12(5), 3045–3050. DOI:10.3892/ol.2016.5110.
- ↑ 4.0 4.1 4.2 Hauseman, Z.J., Fodor, M., Dhembi, A. et al. Structure of the MRAS–SHOC2–PP1C phosphatase complex. Nature 609, 416–423 (2022). doi: 10.1038/s41586-022-05086-1. DOI:10.1038/s41586-022-05086-1.
- ↑ 5.0 5.1 Kwon, J. J., & Hahn, W. C. A Leucine-Rich Repeat Protein Provides a SHOC2 the RAS Circuit: a Structure-Function Perspective. Molecular and cellular biology, 41(4), e00627-20 (2021). doi:10.1128/MCB.00627-20. DOI: 10.1128/MCB.00627-20.
- ↑ Zhou, Y., Prakash, P., Liang, H., et al. Lipid-Sorting Specificity Encoded in K-Ras Membrane Anchor Regulates Signal Output. Cell, 168(1-2), 239–251.e16 doi: 10.1016/j.cell.2016.11.059. DOI: 10.1016/j.cell.2016.11.059.
- ↑ Young, L., Rodriguez-Viciana, P. MRAS: A Close but Understudied Member of the RAS Family. Cold Spring Harbor Perspectives in Medicine (2018). doi: 10.1101/cshperspect.a033621. DOI: 0.1101/cshperspect.a033621.
- ↑ Daniel A. Bonsor, Patrick Alexander, Kelly Snead, Nicole Hartig, Matthew Drew, Simon Messing, Lorenzo I. Finci, Dwight V. Nissley, Frank McCormick, Dominic Esposito, Pablo Rodrigiguez-Viciana, Andrew G. Stephen, Dhirendra K. Simanshu. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. bioRxiv. 2022.05.10.491335. doi: 10.1101/2022.05.10.491335. DOI:10.1101/2022.05.10.491335.
- ↑ 9.0 9.1 9.2 9.3 9.4 Kwon, J.J., Hajian, B., Bian, Y. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).doi: 10.1038/s41586-022-04928-2. DOI:10.1038/s41586-022-04928-2
- ↑ 10.0 10.1 10.2 Kwon, J., Jajian, B., Bian, Y. et al. Comprehensive structure-function evaluation of the SHOC2 holophosphatase reveals disease mechanisms and therapeutic opportunities. In: Proceedings of the American Association for Cancer Research Annual Meeting 2022. DOI: 10.1158/1538-7445.AM2022-LB029.
- ↑ 11.0 11.1 Liau NPD, Johnson MC, Izadi S, Gerosa L, Hammel M, Bruning JM, Wendorff TJ, Phung W, Hymowitz SG, Sudhamsu J. Structural basis for SHOC2 modulation of RAS signalling. Nature. 2022 Jun 29. pii: 10.1038/s41586-022-04838-3. doi:, 10.1038/s41586-022-04838-3. PMID:35768504 doi:http://dx.doi.org/10.1038/s41586-022-04838-3
- ↑ 12.0 12.1 Lavoie, H., Therrien, M. Structural keys unlock RAS–MAPK cellular signaling pathway. Nature 609, 248-249 (2022). doi: 10.1038/d41586-022-02189-7. DOI:10.1038/d41586-022-02189-7.
- ↑ 13.0 13.1 van der Burgt, I. Noonan syndrome. Orphanet J Rare Dis 2, 4 (2007). doi: 10.1186/1750-1172-2-4 DOI: 10.1186/1750-1172-2-4.