We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1790
From Proteopedia
(Difference between revisions)
| Line 14: | Line 14: | ||
[[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 1:'''Active site of PP1C on SMP.</div></font>]] | [[Image:ActiveSiteProto.png|500 px|thumb|'''Figure 1:'''Active site of PP1C on SMP.</div></font>]] | ||
==MRAS== | ==MRAS== | ||
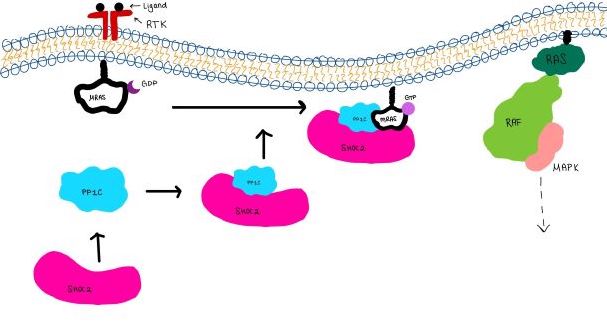
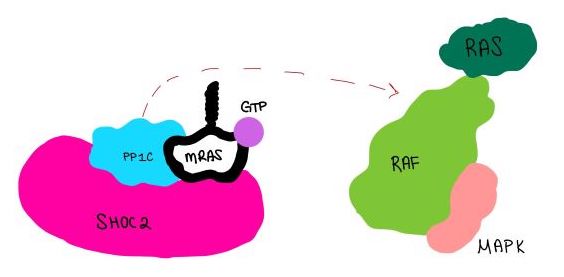
| - | <scene name='95/952717/Mras/2'>MRAS</scene> is a GTPase membrane-bound structure that aids the complex in localizing near other structures such as the RAS-RAF-MAPK complex in order to initiate downstream signaling. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. This conformational change activates the protein allowing it to bind with the SHOC2-PP1C complex. Without the conformational change when GDP is exchanged to GTP, the GDP-MRAS wouldn't be able to bind to SHOC2 because of steric clashing. In comparison to other RAS proteins, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. MRAS engages the SHOC2-PP1C complex and RAF on the same surface indicating that for RAF signaling two separate active MRASs are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. Since MRAS has GTPase activity it can regulate itself by dephosphorylating GTP to GDP to inactivate the protein. | ||
| - | |||
| - | |||
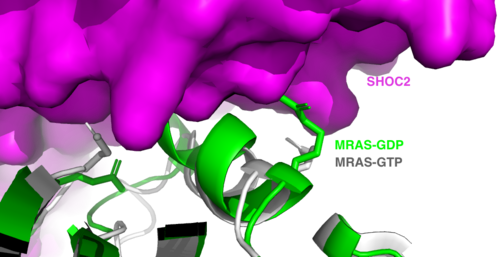
<scene name='95/952717/Mras/2'>MRAS</scene> is a monomeric GTPase. MRAS is membrane-bound due to post-translational lipidation which allows the protein to interact with the inner membrane leaflet. <ref name="Seabra">PMID:9607139</ref> MRAS localizes the SMP complex near RAF and other components of downstream signaling. The region of MRAS not directly bound to the membrane binds SHOC2 and PP1C to orient the complex such that PP1C’s active site faces the serine that will get dephosphorylated on RAF. MRAS also controls SMP complex formation in connection with extracellular signaling based on its dualistic switching between its inactive and active state. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP when a ligand binds to the RTK <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. These regions are the major binding sites with SHOC2. This conformational change activates MRAS allowing it to bind with the SHOC2-PP1C complex. In its inactive GDP-bound state, MRAS is sterically occluded from binding SHOC2. For example, R83 of GDP-bound MRAS directly clashes with SHOC2 as shown in figure 2. In comparison to other RAS proteins such as H/K/NRAS, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. This indicates that the specific structure of MRAS is necessary for SMP function. While MRAS engages the SHOC2-PP1C complex to bring the complex to the membrane, an additional membrane-bound RAS binds RAF nearby. This binding is also stimulated by ligand binding to the RTK. This indicates that for full RAF activation and continuous signaling of Raf, two separate active RAS proteins are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. To inactivate Raf signaling, MRAS uses its intrinsic GTPase to remove the activating gamma-phosphate on GTP. In the GDP-bound state, switch I and II move to the position shown in green in Figure 2. This inactivates SHOC2 binding due to steric clashing which causes the SMP structure to dissociate. | <scene name='95/952717/Mras/2'>MRAS</scene> is a monomeric GTPase. MRAS is membrane-bound due to post-translational lipidation which allows the protein to interact with the inner membrane leaflet. <ref name="Seabra">PMID:9607139</ref> MRAS localizes the SMP complex near RAF and other components of downstream signaling. The region of MRAS not directly bound to the membrane binds SHOC2 and PP1C to orient the complex such that PP1C’s active site faces the serine that will get dephosphorylated on RAF. MRAS also controls SMP complex formation in connection with extracellular signaling based on its dualistic switching between its inactive and active state. In its inactive state, MRAS is bound to GDP. When signaled by growth factors, the GDP is exchanged for GTP when a ligand binds to the RTK <ref name="Hauseman" />. The now <scene name='95/952718/Zoom_in_gtp/1'>GTP bound MRAS</scene> undergoes a conformational change of the <scene name='95/952716/Ras-switch-zoomed/1'>switch I and switch II regions</scene>. These regions are the major binding sites with SHOC2. This conformational change activates MRAS allowing it to bind with the SHOC2-PP1C complex. In its inactive GDP-bound state, MRAS is sterically occluded from binding SHOC2. For example, R83 of GDP-bound MRAS directly clashes with SHOC2 as shown in figure 2. In comparison to other RAS proteins such as H/K/NRAS, MRAS has a greater affinity for the SHOC2-PP1C complex<ref name=”Kubicek”>Kubicek M, Pacher M, Abraham D, Podar K, Eulitz M, Baccarini M. Dephosphorylation of Ser-259 regulates Raf-1 membrane association. J Biol Chem. 2002 Mar 8;277(10):7913-9. [http://10.1074/jbc.M108733200 doi: 10.1074/jbc.M108733200.]</ref>. This indicates that the specific structure of MRAS is necessary for SMP function. While MRAS engages the SHOC2-PP1C complex to bring the complex to the membrane, an additional membrane-bound RAS binds RAF nearby. This binding is also stimulated by ligand binding to the RTK. This indicates that for full RAF activation and continuous signaling of Raf, two separate active RAS proteins are needed. Having two MRASs also help with the co-localization of PP1C to the NTpS region on RAF. To inactivate Raf signaling, MRAS uses its intrinsic GTPase to remove the activating gamma-phosphate on GTP. In the GDP-bound state, switch I and II move to the position shown in green in Figure 2. This inactivates SHOC2 binding due to steric clashing which causes the SMP structure to dissociate. | ||
Revision as of 22:39, 18 April 2023
| |||||||||||