BASIL2023GV1ZBS
From Proteopedia
| Line 30: | Line 30: | ||
==''' Substrate Possibilities '''== | ==''' Substrate Possibilities '''== | ||
[[Image:Substrate_image_2.PNG]] | [[Image:Substrate_image_2.PNG]] | ||
| + | |||
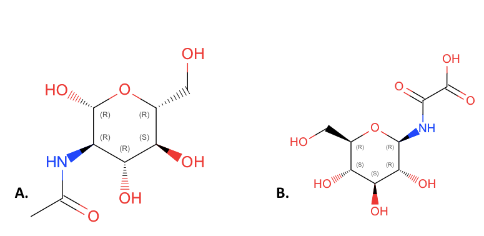
This figure compares the structures of NAG(A) and 4GP(B). All of the components of NAG are present in 4GP but they have been rearranged and an additional carbonyl group has been added to 4GP. We choose NAG as our substrate over 4GP because it is an inhibitor which will not show function. | This figure compares the structures of NAG(A) and 4GP(B). All of the components of NAG are present in 4GP but they have been rearranged and an additional carbonyl group has been added to 4GP. We choose NAG as our substrate over 4GP because it is an inhibitor which will not show function. | ||
== '''Kinase Assays''' == | == '''Kinase Assays''' == | ||
Revision as of 17:40, 19 April 2023
Contents |
Inquiry of the Possible Function of Protein 1ZBS
Abstract
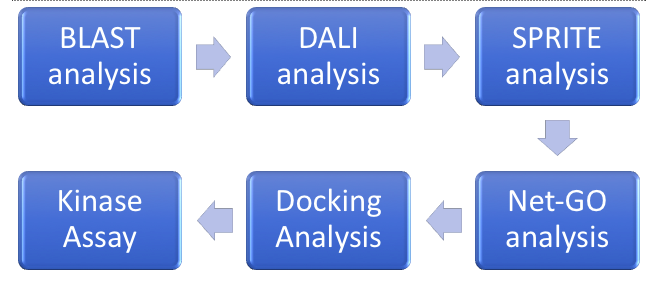
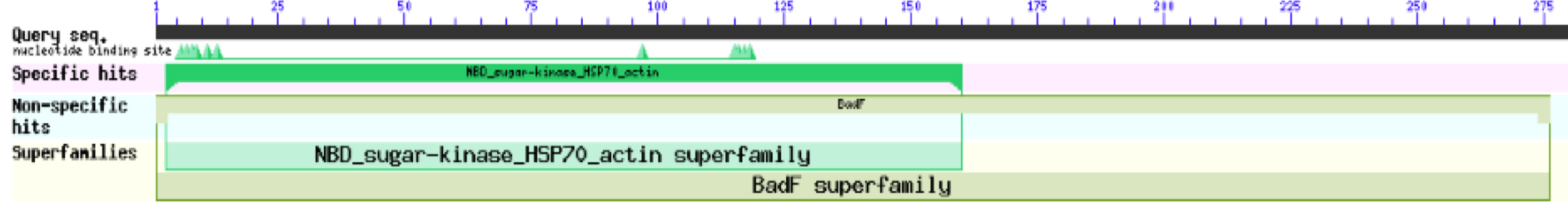
ZBS is a novel protein whose structure is solved but the function is unknown. This research was designed to attempt to uncover the function. Computational research indicated that N-acetylglucosamine (NAG) may be the potential substrate, and that the protein may phosphorylate NAG. This was determined using multiple computational tools, such as BLAST-P, DALI, SPRITE, InterPro. Molecular docking using NAG as a substrate was done with PyMol and Vina docking. After the computational research was completed, the protein was over-expressed and purified. The protein was used to test for activity with the substrate NAG. The kinase assay concluded that NAG is most likely not the substrate for 1ZBS due to a lack of specific activity.
Introduction
| |||||||||||
References
Proteopedia Page Contributors and Editors (what is this?)
Danielle Selover, Carmen Almendarez Rodriguez, Bonnie Hall, Jaime Prilusky