This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1785
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
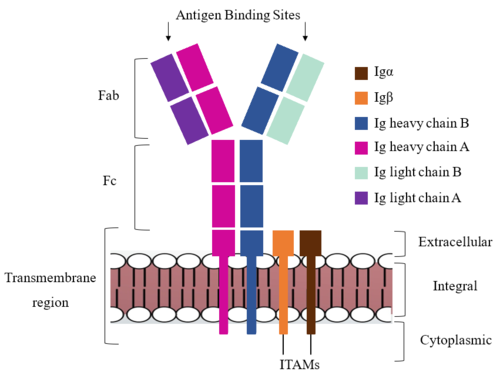
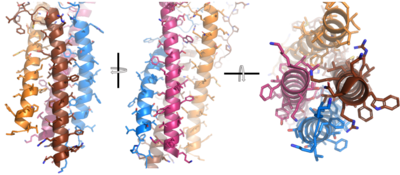
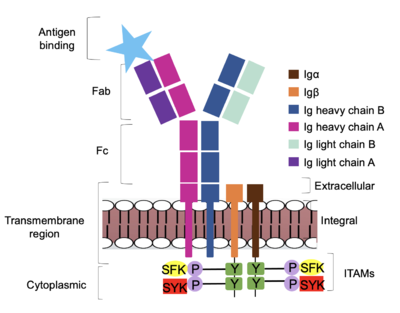
<scene name='95/952714/Extracellular_transmembrane_v2/1'>Extracellular transmembrane region interactions</scene> help hold the heavy chains and <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> chains together in the extracellular portion of the transmembrane region. | <scene name='95/952714/Extracellular_transmembrane_v2/1'>Extracellular transmembrane region interactions</scene> help hold the heavy chains and <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> chains together in the extracellular portion of the transmembrane region. | ||
| - | Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> make a <scene name='95/952713/ | + | Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> make a <scene name='95/952713/Disulfide_bridge/2'>disulfide bridge</scene> to stabilize the IgM-BCR and drive downstream signaling. |
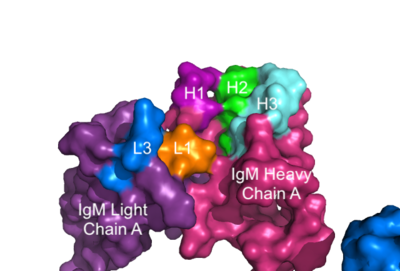
To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a stabilizing <scene name='95/952713/Heavy_interface/5'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref> | To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a stabilizing <scene name='95/952713/Heavy_interface/5'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref> | ||
Revision as of 20:54, 20 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
- ↑ Sathe A, Cusick JK. Biochemistry, Immunoglobulin M. 2022 Dec 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32310455. https://pubmed.ncbi.nlm.nih.gov/32310455/
- ↑ 2.0 2.1 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. doi: 10.1126/science.abo3923. Epub 2022, Aug 18. PMID:35981043 doi:http://dx.doi.org/10.1126/science.abo3923
- ↑ 3.0 3.1 3.2 3.3 Ma X, Zhu Y, Dong, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. doi: 10.1126/science.abo3828. Epub 2022, Aug 18. PMID:35981028 doi:http://dx.doi.org/10.1126/science.abo3828
- ↑ 4.0 4.1 4.2 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. doi: 10.1126/science.add8065. Epub 2022 Aug 18.[http://dx.doi.org/10.1126/science.add8065 DOI:10.1126/science.add8065
- ↑ 5.0 5.1 5.2 Dylke J, Lopes J, Dang-Lawson M, Machtaler S, Matsuuchi L. Role of the extracellular and transmembrane domain of Ig-alpha/beta in assembly of the B cell antigen receptor (BCR). Immunol Lett. 2007 Sep 15;112(1):47-57. doi: 10.1016/j.imlet.2007.06.005. Epub 2007 Jul 23. [http://dx.doi.org/10.1016/j.imlet.2007.06.005 DOI:10.1016/j.imlet.2007.06.005
- ↑ Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811-7. Epub 2010 Jul 8. PMID:20616231 doi:10.1126/science.1192819
Student Contributors
DeTonyeá Dickson, Allison Goss, Jackson Payton