We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 37: | Line 37: | ||
|} | |} | ||
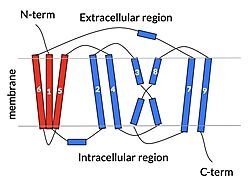
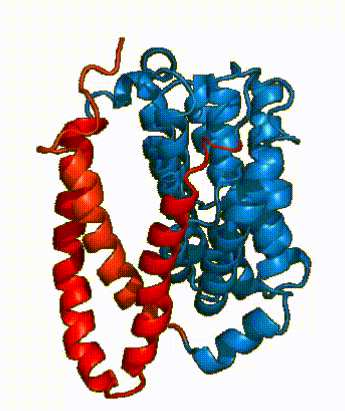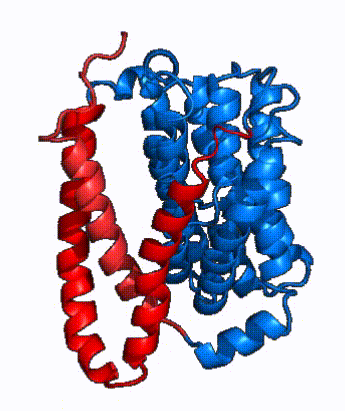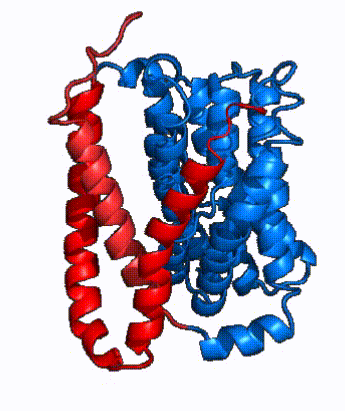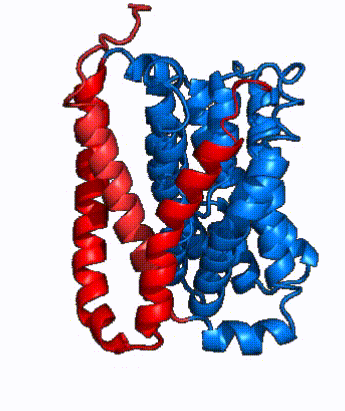
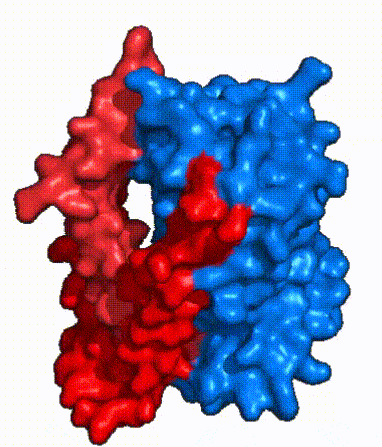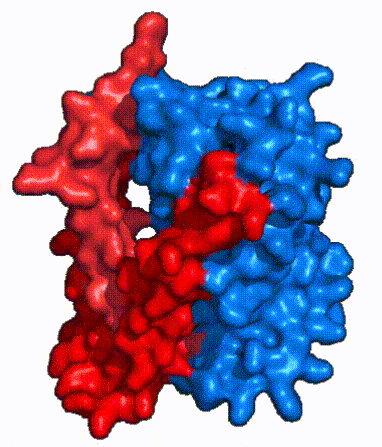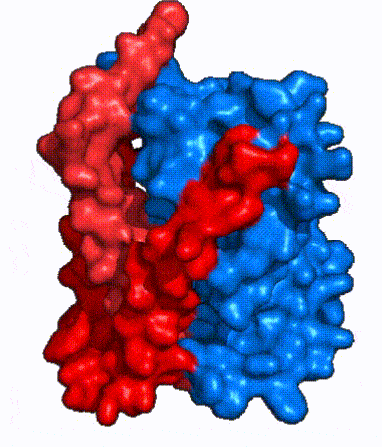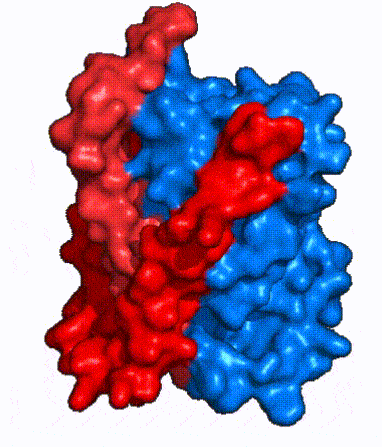
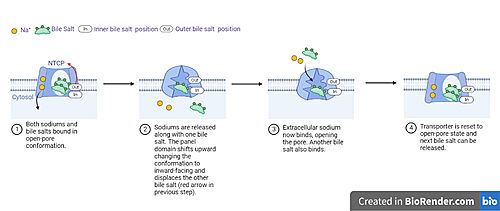
| - | In order to reveal these binding sites to initiate bile salt transport, NTCP exists in two different conformations; the <scene name='95/952722/Open_pore_conf/4'>open pore conformation</scene> and the <scene name='95/952722/Inward_facing_conf/1'>inward facing conformation</scene>. <ref name="Goutam"/> NTCP undergoes a conformational change from inward facing to open pore which exposes the binding sites to the extracellular region to allow the sodium ions and bile salts to bind. NTCP utilizes an [https://www.sciencedirect.com/science/article/pii/S0092867417302891 elevator-alternating mechanism] <Ref name = "Latorraca"> Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107. </ref> where one domain <font color=' | + | In order to reveal these binding sites to initiate bile salt transport, NTCP exists in two different conformations; the <scene name='95/952722/Open_pore_conf/4'>open pore conformation</scene> and the <scene name='95/952722/Inward_facing_conf/1'>inward facing conformation</scene>. <ref name="Goutam"/> NTCP undergoes a conformational change from inward facing to open pore which exposes the binding sites to the extracellular region to allow the sodium ions and bile salts to bind. NTCP utilizes an [https://www.sciencedirect.com/science/article/pii/S0092867417302891 elevator-alternating mechanism] <Ref name = "Latorraca"> Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107. </ref> where one domain <font color='red'><b>(panel)</b></font> does most of the translocation, and the other domain <font color='#6060ff'><b>(core)</b></font> remains stationary. <Ref name = "Asami"> Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4 </ref> In this movement, the <scene name='95/952722/Ntcp_core_domain-_blue/8'>core domain</scene> and the <scene name='95/952722/Ntcp_panel_domain-_red/4'>panel domain</scene> rotate 20° with the <font color='red'><b>panel domain</b></font> moving 5 Å away from the <font color='#6060ff'><b>core domain</b></font>, which remains relatively rigid. This conformational change reveals the two sodium ion binding sites as well as the <scene name='95/952721/Amphipathic_patterns/2'>amphipathic pore</scene> in the membrane. The movement of the panel domain is facilitated by <scene name='95/952722/Pro_and_gly_hinges/5'>proline and glycine residues</scene> located in the <scene name='95/952722/Connector_helices/6'>connector helices</scene> between the panel and core domains. <scene name='95/952722/Pro_and_gly_hinges/7'>These residues</scene> <font color='#FCE205'><b>(yellow)</b></font> act as hinges that assist in the movement of the panel domain away from the core domain. <ref name="Goutam"/> |
== Bile Salt Transport == | == Bile Salt Transport == | ||
Revision as of 22:02, 20 April 2023
Contents |
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ 4.0 4.1 4.2 4.3 4.4 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 5.0 5.1 5.2 5.3 5.4 Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107.
- ↑ 8.0 8.1 8.2 8.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
- ↑ Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082.
- ↑ 10.0 10.1 Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259.
PDB Files
Bile salts and sodium ions bound: 7zyi Open-pore NTCP: 7pqq Inward-facing NTCP: 7pqg
Student Contributors
- Isabelle White
- Lena Barko