We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1794
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
==== Sodium ==== | ==== Sodium ==== | ||
| - | NTCP, like other SLC10 family members, | + | One of NTCP's key structural features, like other SLC10 family members, is <scene name='95/952721/Sodium_binding/5'>two sodium binding sites</scene>. Many polar and negatively charged residues (68, 105, 106, 119, 123, 257, 261) form ion-dipole or dipole-dipole interactions with the sodium ions in these sites with a high level of conservation, suggesting sodium binding is coupled to bile salt transport. <Ref name = "Goutam"/> Mutations in the X-motif near sodium binding sites also inhibit bile salt transport function, suggesting that sodium is required for salt binding. |
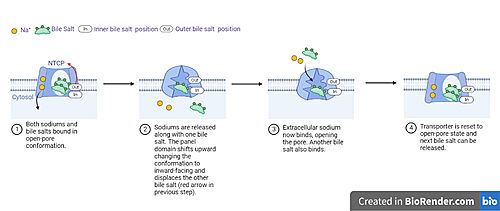
<Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> Sodium transport facilitates structural changes in NTCP from its typical open-pore state to an inward-facing (closed-pore) state. The inward-facing state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. <Ref name = "Goutam"/> Gating of the channel with sodium in this way allows for sodium concentrations to regulate uptake of taurocholates. <Ref name = "Goutam"/> When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> Overall, this suggests that thermodynamically favorable sodium transport is coupled to moving bile salts against their concentration gradient. <Ref name = "Liu"/> | <Ref name = "Goutam"> Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). [https://doi.org/10.1038/s41586-022-04723-z DOI: 10.1038/s41586-022-04723-z]. </Ref> Sodium transport facilitates structural changes in NTCP from its typical open-pore state to an inward-facing (closed-pore) state. The inward-facing state is favored in the absence of sodium ions, while open-pore state is favored in the presence of sodium ions. <Ref name = "Goutam"/> Gating of the channel with sodium in this way allows for sodium concentrations to regulate uptake of taurocholates. <Ref name = "Goutam"/> When intracellular sodium levels are higher, open-pore state is favored allowing for the diffusion of taurocholates. However, when extracellular sodium levels are high, inward-facing state is favored preventing diffusion of taurocholates. <ref name="Goutam"/> Overall, this suggests that thermodynamically favorable sodium transport is coupled to moving bile salts against their concentration gradient. <Ref name = "Liu"/> | ||
==== Bile Salts ==== | ==== Bile Salts ==== | ||
| - | + | Another key feature of NTCP is its <scene name='95/952722/Amphipathic_patterns/1'>amphipathic pore</scene> which allows for bile salt transport across the hydrophilic membrane. The pore surface remains {{Template:ColorKey_Hydrophobic}}, while the <scene name='95/952721/Amphipathic_patterns_pore/2'>lining of the open pore</scene> is largely {{Template:ColorKey_Polar}}. In the inward-facing or closed-pore conformation, the polar pore residues are inaccessible. Only the surface hydrophobic residues are exposed. As the pore opens up, inner polar residues become accessible allowing for the binding of hydrophilic bile salts. The pattern of hydrophobic and polar residues within the pore matches the amphipathic patterns within taurocholates, [https://en.wikipedia.org/wiki/Steroid steroids], and [https://en.wikipedia.org/wiki/Thyroid_hormones thyroid hormones]. <Ref name = Qi> Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294 </ref> Using this amphipathic pore, provides the channel with specificity while preventing leakage of other substrates. Essential <scene name='95/952722/Bile_salts_res/1'>bile salt binding residues</scene> form Van der Waals interactions with bile salt substrates, while others form dipole-dipole or ionic interactions. The core domain contributes most of the polar domains, while the panel domain contributes mainly hydrophobic surface. | |
=== Conformational Change === | === Conformational Change === | ||
| Line 47: | Line 47: | ||
== HBV Binding and Infection== | == HBV Binding and Infection== | ||
| - | NTCP is the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV and HDV. <Ref name = "Asami"/>These viruses are known to use <scene name='95/952721/Hep_patches/2'>two different patches</scene> <font color='#00e080'><b>(residues 84-87 and 157-165)</b></font> on NTCP for binding and entry. The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through the <scene name='95/952721/Hbv_patch_1/1'>first hydrophobic patch</scene> on NTCP containing <font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the bile salt transport tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> The <scene name='95/952721/Hbv_patch_2/1'>other hydrophobic patch</scene> consisting of <font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that do not inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> This S267F mutation is a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism], where a change in one nucleotide in the sequence has caused a lack of bile salt transport activity or viral infection. <Ref name = "Park"/> It is hypothesized that due to the lack of bile salt transport in this mutation that the open-pore state during bile salt transport is necessary for HBV and HDV infection, suggesting the two functionally overlap. <Ref name = "Park"/> | + | The other main function of NTCP is its role as the only [https://rupress.org/jcb/article/195/7/1071/54877/The-cell-biology-of-receptor-mediated-virus entry receptor] <Ref name = "Grove"> Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082. </ref> into the liver for HBV and HDV. <Ref name = "Asami"/>These viruses are known to use <scene name='95/952721/Hep_patches/2'>two different patches</scene> <font color='#00e080'><b>(residues 84-87 and 157-165)</b></font> on NTCP for binding and entry. The [https://en.wikipedia.org/wiki/Myristoylation myristolated] PreS1 domain of HBV binds to NTCP through the <scene name='95/952721/Hbv_patch_1/1'>first hydrophobic patch</scene> on NTCP containing <font color='#00e080'><b>residues 157-165</b></font> on the open pore surface. <Ref name = "Asami"/> These residues form part of the bile salt transport tunnel resulting in HBV binding and bile salt transport directly competing and interfering with one another. <Ref name = "Asami"/> The <scene name='95/952721/Hbv_patch_2/1'>other hydrophobic patch</scene> consisting of <font color='#00e080'><b>residues 84-87</b></font> found on the N-terminus of NTCP does not overlap with bile salt binding and may be used for the development of [https://en.wikipedia.org/wiki/Antiviral_drug antivirals] that do not inhibit bile uptake <Ref name = "Park"/>. Other minor variations within NTCP provide species specificity for HBV or virus resistance, such as mutant S267F found in East Asia. <Ref name = "Park"/> This S267F mutation is a [https://en.wikipedia.org/wiki/Single-nucleotide_polymorphism single-nucleotide polymorphism], where a change in one nucleotide in the sequence has caused a lack of bile salt transport activity or viral infection. <Ref name = "Park"/> It is hypothesized that due to the lack of bile salt transport in this mutation that the open-pore state during bile salt transport is necessary for HBV and HDV infection, suggesting the two functionally overlap. <Ref name = "Park"/> |
The exact mechanism by which NTCP mediates viral internalization is still being determined; however, current evidence suggests it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound, the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. HBV may also interact with other receptors or host cell factors, as cells overexpressing NTCP alone had low infection efficiency. <Ref name = "Herrscher"/> | The exact mechanism by which NTCP mediates viral internalization is still being determined; however, current evidence suggests it works through [https://en.wikipedia.org/wiki/Viral_entry#Entry_via_endocytosis endocytosis.] <Ref name = "Herrscher"> Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259. </ref> Once HBV is bound, the NTCP/HBV complex is taken into the cell where viral contents are dumped into the cytoplasm to then begin [https://en.wikipedia.org/wiki/Viral_replication viral replication]. HBV may also interact with other receptors or host cell factors, as cells overexpressing NTCP alone had low infection efficiency. <Ref name = "Herrscher"/> | ||
Current revision
Contents |
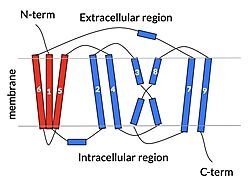
Sodium Taurocholate Co-Transporting Polypeptide
| |||||||||||
References
- ↑ Stieger B. The role of the sodium-taurocholate cotransporting polypeptide (NTCP) and of the bile salt export pump (BSEP) in physiology and pathophysiology of bile formation. Handb Exp Pharmacol. 2011;(201):205-59. doi: 10.1007/978-3-642-14541-4_5. PMID: 21103971. DOI: DOI: 10.1007/978-3-642-14541-4_5.
- ↑ Geyer, J., Wilke, T. & Petzinger, E. The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships. Naunyn Schmied Arch Pharmacol 372, 413–431 (2006). https://doi.org/10.1007/s00210-006-0043-8
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 Goutam, K., Ielasi, F.S., Pardon, E. et al. Structural basis of sodium-dependent bile salt uptake into the liver. Nature 606, 1015–1020 (2022). DOI: 10.1038/s41586-022-04723-z.
- ↑ 4.0 4.1 4.2 4.3 4.4 Park, JH., Iwamoto, M., Yun, JH. et al. Structural insights into the HBV receptor and bile acid transporter NTCP. Nature 606, 1027–1031 (2022). https://doi.org/10.1038/s41586-022-04857-0.
- ↑ 5.0 5.1 5.2 5.3 5.4 Liu, H., Irobalieva, R.N., Bang-Sørensen, R. et al. Structure of human NTCP reveals the basis of recognition and sodium-driven transport of bile salts into the liver. Cell Res 32, 773–776 (2022). https://doi.org/10.1038/s41422-022-00680-4
- ↑ Qi X. and Li W. (2022). Unlocking the secrets to human NTCP structure. The Innovation 3(5), 100294. https://doi.org/10.1016/j.xinn.2022.100294
- ↑ Latorraca, N. R.; Fastman, N. M.; Venkatakrishnan, A. J.; Frommer, W. B.; Dror, R. O.; Feng, L. Mechanism of Substrate Translocation in an Alternating Access Transporter. Cell 2017, 169 (1), 96–107.
- ↑ 8.0 8.1 8.2 8.3 Asami, J., Kimura, K.T., Fujita-Fujiharu, Y. et al.Structure of the bile acid transporter and HBV receptor NTCP. Nature 606, 1021–1026 (2022). https://doi.org/10.1038/s41586-022-04845-4
- ↑ Grove, J.; Marsh, M. The Cell Biology of Receptor-Mediated Virus Entry. Journal of Cell Biology 2011, 195 (7), 1071–1082.
- ↑ 10.0 10.1 Herrscher C, Roingeard P, Blanchard E. Hepatitis B Virus Entry into Cells. Cells. 2020 Jun 18;9(6):1486. doi: 10.3390/cells9061486. PMID: 32570893; PMCID: PMC7349259.
PDB Files
Student Contributors
- Isabelle White
- Lena Barko