We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1786
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | |||
| - | ---- | ||
='''Human B-cell Antigen Receptor: IgM BCR'''= | ='''Human B-cell Antigen Receptor: IgM BCR'''= | ||
| Line 29: | Line 27: | ||
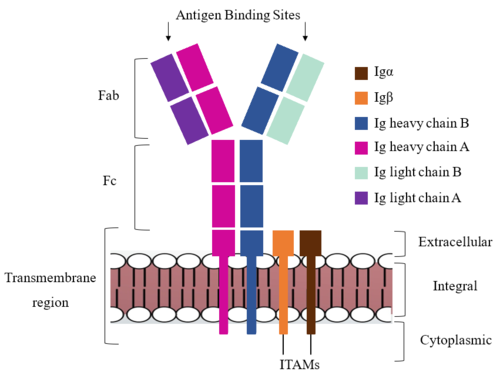
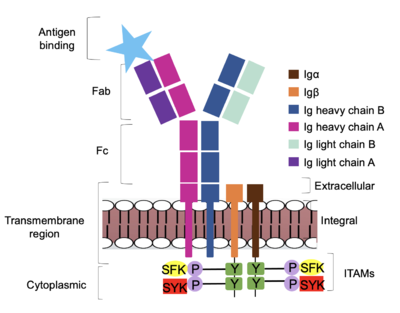
Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). <ref name="Ma">PMID:35981028</ref> The structures of the ITAM regions have not yet been determined. | Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). <ref name="Ma">PMID:35981028</ref> The structures of the ITAM regions have not yet been determined. | ||
| + | |||
| + | ---- | ||
===Fc Region=== | ===Fc Region=== | ||
| Line 39: | Line 39: | ||
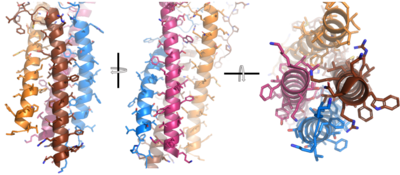
To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a <scene name='95/952713/Trans_heavy/7'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref> | To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a <scene name='95/952713/Trans_heavy/7'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref> | ||
| + | |||
| + | ---- | ||
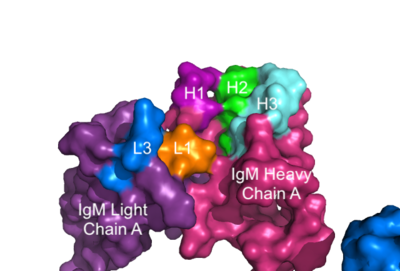
===Fab Region=== | ===Fab Region=== | ||
| Line 50: | Line 52: | ||
{{Clear}} | {{Clear}} | ||
| + | ---- | ||
=='''Signal Transduction'''== | =='''Signal Transduction'''== | ||
Revision as of 23:53, 20 April 2023
Human B-cell Antigen Receptor: IgM BCR
| |||||||||||
References
- ↑ Sathe A, Cusick JK. Biochemistry, Immunoglobulin M. 2022 Dec 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32310455. https://pubmed.ncbi.nlm.nih.gov/32310455/
- ↑ 2.0 2.1 Su Q, Chen M, Shi Y, Zhang X, Huang G, Huang B, Liu D, Liu Z, Shi Y. Cryo-EM structure of the human IgM B cell receptor. Science. 2022 Aug 19;377(6608):875-880. doi: 10.1126/science.abo3923. Epub 2022, Aug 18. PMID:35981043 doi:http://dx.doi.org/10.1126/science.abo3923
- ↑ 3.0 3.1 3.2 3.3 Ma X, Zhu Y, Dong, Chen Y, Wang S, Yang D, Ma Z, Zhang A, Zhang F, Guo C, Huang Z. Cryo-EM structures of two human B cell receptor isotypes. Science. 2022 Aug 19;377(6608):880-885. doi: 10.1126/science.abo3828. Epub 2022, Aug 18. PMID:35981028 doi:http://dx.doi.org/10.1126/science.abo3828
- ↑ 4.0 4.1 4.2 Tolar P, Pierce SK. Unveiling the B cell receptor structure. Science. 2022 Aug 19;377(6608):819-820. doi: 10.1126/science.add8065. Epub 2022 Aug 18.[http://dx.doi.org/10.1126/science.add8065 DOI:10.1126/science.add8065
- ↑ 5.0 5.1 5.2 Dylke J, Lopes J, Dang-Lawson M, Machtaler S, Matsuuchi L. Role of the extracellular and transmembrane domain of Ig-alpha/beta in assembly of the B cell antigen receptor (BCR). Immunol Lett. 2007 Sep 15;112(1):47-57. doi: 10.1016/j.imlet.2007.06.005. Epub 2007 Jul 23. [http://dx.doi.org/10.1016/j.imlet.2007.06.005 DOI:10.1016/j.imlet.2007.06.005
- ↑ Daniels R, Kurowski B, Johnson AE, Hebert DN. N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell. 2003 Jan;11(1):79-90. PMID:12535523 doi:10.1016/s1097-2765(02)00821-3
- ↑ Bakshi T, Pham D, Kaur R, Sun B. Hidden Relationships between N-Glycosylation and Disulfide Bonds in Individual Proteins. Int J Mol Sci. 2022 Mar 29;23(7):3742. PMID:35409101 doi:10.3390/ijms23073742
- ↑ Mirazimi A, Svensson L. Carbohydrates facilitate correct disulfide bond formation and folding of rotavirus VP7. J Virol. 1998 May;72(5):3887-92. PMID:9557673 doi:10.1128/JVI.72.5.3887-3892.1998
- ↑ Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Do Kwon Y, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811-7. Epub 2010 Jul 8. PMID:20616231 doi:10.1126/science.1192819
Student Contributors
DeTonyeá Dickson, Allison Goss, Jackson Payton