|
|
| Line 5: |
Line 5: |
| | =='''Introduction'''== | | =='''Introduction'''== |
| | | | |
| - | <div style="text-align: justify">The [https://en.wikipedia.org/wiki/Adaptive_immune_system adaptive immune response] possessed by [https://en.wikipedia.org/wiki/Vertebrate vertebrate] animals owes much of its function to [https://en.wikipedia.org/wiki/B_cell B cells]. These specialized immune cells produce [https://en.wikipedia.org/wiki/Antibody antibodies] and immunoglobulins (Ig), the membrane bound equivalent to antibodies. B cells can produce a variety of Ig compounds including [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD], and [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM]. These antibodies and Ig compounds bind to specific compounds called [https://en.wikipedia.org/wiki/Antigen antigens]. When an IgM combines with a [https://en.wikipedia.org/wiki/B-cell_receptor B cell receptor] (BCR), it can then send a signal in the form of a conformational change through the B cell membrane to stimulate the production of more antibodies that recognize that antigen. <ref name="Sathe">Sathe A, Cusick JK. Biochemistry, Immunoglobulin M. 2022 Dec 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32310455. https://pubmed.ncbi.nlm.nih.gov/32310455/</ref>
| + | The [https://en.wikipedia.org/wiki/Adaptive_immune_system adaptive immune response] possessed by [https://en.wikipedia.org/wiki/Vertebrate vertebrate] animals owes much of its function to [https://en.wikipedia.org/wiki/B_cell B cells]. These specialized immune cells produce [https://en.wikipedia.org/wiki/Antibody antibodies] and immunoglobulins (Ig), the membrane bound equivalent to antibodies. B cells can produce a variety of Ig compounds including [https://en.wikipedia.org/wiki/Immunoglobulin_G IgG], [https://en.wikipedia.org/wiki/Immunoglobulin_A IgA], [https://en.wikipedia.org/wiki/Immunoglobulin_E IgE], [https://en.wikipedia.org/wiki/Immunoglobulin_D IgD], and [https://en.wikipedia.org/wiki/Immunoglobulin_M IgM]. These antibodies and Ig compounds bind to specific compounds called [https://en.wikipedia.org/wiki/Antigen antigens]. When an IgM combines with a [https://en.wikipedia.org/wiki/B-cell_receptor B cell receptor] (BCR), it can then send a signal in the form of a conformational change through the B cell membrane to stimulate the production of more antibodies that recognize that antigen. <ref name="Sathe">Sathe A, Cusick JK. Biochemistry, Immunoglobulin M. 2022 Dec 19. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32310455. https://pubmed.ncbi.nlm.nih.gov/32310455/</ref> |
| | | | |
| - | <div style="text-align: justify">The structure of the IgM BCR complex was determined by two research groups using [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo EM]. They also determined the structure of IgG. <ref name="Su">PMID:35981043</ref>, <ref name="Ma">PMID:35981028</ref>
| + | The structure of the IgM BCR complex was determined by two research groups using [https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryo EM]. They also determined the structure of IgG. <ref name="Su">PMID:35981043</ref>, <ref name="Ma">PMID:35981028</ref> |
| | | | |
| | =='''Structure'''== | | =='''Structure'''== |
| Line 18: |
Line 18: |
| | ===Transmembrane Region=== | | ===Transmembrane Region=== |
| | | | |
| - | <div style="text-align: justify">The IgM BCR is anchored to [https://en.wikipedia.org/wiki/B_cell B-cell] membranes through the <scene name='95/952714/Integral_region/15'>transmembrane region</scene> which is broken up into both extracellular and integral domains which sit on top of or span through the membrane, respectively (Figure 1). The extracellualr region is primarily composed of [https://proteopedia.org/wiki/index.php/Beta_sheet β-sheets]while the integral region is composed of [https://proteopedia.org/wiki/index.php/Alpha_helix#:~:text=An%20alpha%20helix%20is%20a,can%20be%20of%20arbitrary%20length. α-helices]. IgM BCR assembly requires dimerization of the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> subunits which embed within the B-cell membrane. <ref name="Tolar"/> The <scene name='95/952714/Ig_alpha_beta/5'>Igα and Igβ heterodimer</scene> dimerizes within the extracellular region with a <scene name='95/952714/Extracellular_disulfide_bridge/6'>disulfide bridge</scene>. Additional dimerization occurs within the integral region via a hydrogen bond; the residues involved have not been confirmed. Although the mechanism of disulfide bridge formation is still unknown, <scene name='95/952714/Extracellular_glycosylation/2'>glycosylation</scene> via [https://en.wikipedia.org/wiki/N-linked_glycosylation N-linked asparagine glycosyl groups] <b><span class="text-lightgreen">(NAGs)</span></b> in the extracellular region of both the <b><span class="text-brown">Igα</span></b> and and <b><span class="text-orange">Igβ</span></b> chains is hypothesized to facilitate this process. The NAG groups are believed to be essential for the recruitment of [https://en.wikipedia.org/wiki/Chaperone_(protein) Chaperone proteins] to optimize the folding process. <ref name="Daniels">PMID:12535523</ref> Past studies with human and viral proteins have shown that the presence of NAGs not only facilitate the rapid formation of disulfide bridges, but also ensure correct location. <ref name="Bakshi">PMID:35409101</ref>, <ref name="Mirazimi">PMID:9557673</ref> The recruited chaperone proteins will remain bound to the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> subunits until dimerization occurs. <ref name="Dylke"/>
| + | The IgM BCR is anchored to [https://en.wikipedia.org/wiki/B_cell B-cell] membranes through the <scene name='95/952714/Integral_region/15'>transmembrane region</scene> which is broken up into both extracellular and integral domains which sit on top of or span through the membrane, respectively (Figure 1). The extracellualr region is primarily composed of [https://proteopedia.org/wiki/index.php/Beta_sheet β-sheets]while the integral region is composed of [https://proteopedia.org/wiki/index.php/Alpha_helix#:~:text=An%20alpha%20helix%20is%20a,can%20be%20of%20arbitrary%20length. α-helices]. IgM BCR assembly requires dimerization of the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> subunits which embed within the B-cell membrane. <ref name="Tolar"/> The <scene name='95/952714/Ig_alpha_beta/5'>Igα and Igβ heterodimer</scene> dimerizes within the extracellular region with a <scene name='95/952714/Extracellular_disulfide_bridge/6'>disulfide bridge</scene>. Additional dimerization occurs within the integral region via a hydrogen bond; the residues involved have not been confirmed. Although the mechanism of disulfide bridge formation is still unknown, <scene name='95/952714/Extracellular_glycosylation/2'>glycosylation</scene> via [https://en.wikipedia.org/wiki/N-linked_glycosylation N-linked asparagine glycosyl groups] <b><span class="text-lightgreen">(NAGs)</span></b> in the extracellular region of both the <b><span class="text-brown">Igα</span></b> and and <b><span class="text-orange">Igβ</span></b> chains is hypothesized to facilitate this process. The NAG groups are believed to be essential for the recruitment of [https://en.wikipedia.org/wiki/Chaperone_(protein) Chaperone proteins] to optimize the folding process. <ref name="Daniels">PMID:12535523</ref> Past studies with human and viral proteins have shown that the presence of NAGs not only facilitate the rapid formation of disulfide bridges, but also ensure correct location. <ref name="Bakshi">PMID:35409101</ref>, <ref name="Mirazimi">PMID:9557673</ref> The recruited chaperone proteins will remain bound to the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> subunits until dimerization occurs. <ref name="Dylke"/> |
| | | | |
| - | <div style="text-align: justify">After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane and intertwine with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. <ref name="Tolar"/> The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene>, made up by the <b><span class="text-brown">alpha</span></b>, <b><span class="text-orange">beta</span></b>, and heavy chains ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>), are primarily hydrophobic; this allows for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The four helices (Figure 2) are intertwined and primarily held together through interactions between the <scene name='95/952714/Integral_helices_2/3'>hydrophobic side chains</scene>; however, a a few polar residues are included which allow for additional interactions with the polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. <ref name="Dylke"/>
| + | After <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane and intertwine with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. <ref name="Tolar"/> The side chains of this <scene name='95/952714/Integral_helices_2/2'>4-pass integral helix structure</scene>, made up by the <b><span class="text-brown">alpha</span></b>, <b><span class="text-orange">beta</span></b>, and heavy chains ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>), are primarily hydrophobic; this allows for interactions with the hydrophobic tails in the [https://en.wikipedia.org/wiki/Lipid_bilayer phospholipid bilayer]. The four helices (Figure 2) are intertwined and primarily held together through interactions between the <scene name='95/952714/Integral_helices_2/3'>hydrophobic side chains</scene>; however, a a few polar residues are included which allow for additional interactions with the polar residues on the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains. <ref name="Dylke"/> |
| | | | |
| | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' PyMOL image of the integral helices in IgM BCR (PDB:7xq8). The structure is shown rotated on the x and y axes to illustrate how the chains are intertwined. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] | | [[Image:Integral_helix_figure.png|400 px|left|thumb|'''Figure 2. 4-pass integral helix.''' PyMOL image of the integral helices in IgM BCR (PDB:7xq8). The structure is shown rotated on the x and y axes to illustrate how the chains are intertwined. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B.]] |
| | {{Clear}} | | {{Clear}} |
| | | | |
| - | <div style="text-align: justify">Within the transmembrane region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> associate (Figure 1) asymmetrically to facilitate intracellular signaling cascades. The <scene name='95/952713/Trans_heavy/9'>transmembrane heavy chain interface</scene> allows them to pack together via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on '''{{Font color|violet|heavy chain A}}''' donates a hydrogen bond to Ser584 and to Ser588 on <b><span class="text-blue">heavy chain B</span></b>. This creates a [https://en.wikipedia.org/wiki/Hydrogen_bond bifurcated hydrogen bond], essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains within the BCR. <ref name="Su">PMID:35981043</ref>
| + | Within the transmembrane region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> associate (Figure 1) asymmetrically to facilitate intracellular signaling cascades. The <scene name='95/952713/Trans_heavy/9'>transmembrane heavy chain interface</scene> allows them to pack together via [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals] contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on '''{{Font color|violet|heavy chain A}}''' donates a hydrogen bond to Ser584 and to Ser588 on <b><span class="text-blue">heavy chain B</span></b>. This creates a [https://en.wikipedia.org/wiki/Hydrogen_bond bifurcated hydrogen bond], essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains within the BCR. <ref name="Su">PMID:35981043</ref> |
| | | | |
| - | <div style="text-align: justify">Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). <ref name="Ma">PMID:35981028</ref> The structures of the ITAM regions have not yet been determined.
| + | Furthermore, both the <b><span class="text-brown">Igα</span></b> and <b><span class="text-orange">Igβ</span></b> chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an [https://en.wikipedia.org/wiki/Immunoreceptor_tyrosine-based_activation_motif ITAM region] to facilitate signal transduction (Figure 4). <ref name="Ma">PMID:35981028</ref> The structures of the ITAM regions have not yet been determined. |
| | | | |
| | ---- | | ---- |
| Line 33: |
Line 33: |
| | ===Fc Region=== | | ===Fc Region=== |
| | | | |
| - | <div style="text-align: justify">The constant region of IgM is made up of the two <scene name='95/952714/Heavy_chain/1'>heavy chains</scene>. These heavy chains form a bridge connecting the FAB region or variable region to the transmembrane region (Figure 1). They also act as a wire that the variable region can send a signal through to the transmembrane region as a mechanical change.
| + | The constant region of IgM is made up of the two <scene name='95/952714/Heavy_chain/1'>heavy chains</scene>. These heavy chains form a bridge connecting the FAB region or variable region to the transmembrane region (Figure 1). They also act as a wire that the variable region can send a signal through to the transmembrane region as a mechanical change. |
| | | | |
| | <scene name='95/952715/Extracellular_transmembrane_v2/10'>Extracellular transmembrane interactions</scene> help hold the heavy chains and <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> chains together in the extracellular portion of the transmembrane region. | | <scene name='95/952715/Extracellular_transmembrane_v2/10'>Extracellular transmembrane interactions</scene> help hold the heavy chains and <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> chains together in the extracellular portion of the transmembrane region. |
| | | | |
| - | <div style="text-align: justify">Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> make a <scene name='95/952713/Disulfides/5'>disulfide bridge</scene> to stabilize the IgM-BCR and drive downstream signaling.
| + | Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, '''{{Font color|violet|heavy chain A}}''' and <b><span class="text-blue">heavy chain B</span></b> make a <scene name='95/952713/Disulfides/5'>disulfide bridge</scene> to stabilize the IgM-BCR and drive downstream signaling. |
| | | | |
| - | <div style="text-align: justify">To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a <scene name='95/952713/Trans_heavy/7'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref>
| + | To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of '''{{Font color|violet|heavy chain A}}''' makes an asymmetrical association with constant region three of <b><span class="text-blue">heavy chain B</span></b> to create a <scene name='95/952713/Trans_heavy/7'>heavy chain interface</scene>. More specifically, Arg243 and Arg251 residues from '''{{Font color|violet|heavy chain A}}''' donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on <b><span class="text-blue">heavy chain B</span></b>. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. <ref name="Ma">PMID:35981028</ref> |
| | | | |
| | ---- | | ---- |
| Line 45: |
Line 45: |
| | ===Fab Region=== | | ===Fab Region=== |
| | | | |
| - | <div style="text-align: justify">The Fab region of the antibody is where antigen recognition occurs upon binding (Figure 1). On each arm is one heavy ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) and one light (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) chain, both containing domains identical to their respective counterparts. Repeats of β-sandwiches form the [https://en.wikipedia.org/wiki/Antibody constant and variable domains] within the Fab region as antigen recognition occurs at the variable domain while the constant domain connects it to the rest of the IgM complex. Because the Fab region of IgM is poorly resolved, a structural analysis of an HIV neutralizing antibody called VCR01 was performed to approximate where an antigen would bind to at the <scene name='95/952714/Variable_region/1'>variable region</scene>. <ref name="Zhou">PMID:20616231</ref>
| + | The Fab region of the antibody is where antigen recognition occurs upon binding (Figure 1). On each arm is one heavy ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) and one light (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) chain, both containing domains identical to their respective counterparts. Repeats of β-sandwiches form the [https://en.wikipedia.org/wiki/Antibody constant and variable domains] within the Fab region as antigen recognition occurs at the variable domain while the constant domain connects it to the rest of the IgM complex. Because the Fab region of IgM is poorly resolved, a structural analysis of an HIV neutralizing antibody called VCR01 was performed to approximate where an antigen would bind to at the <scene name='95/952714/Variable_region/1'>variable region</scene>. <ref name="Zhou">PMID:20616231</ref> |
| | | | |
| - | <div style="text-align: justify">The IgM-BCR contains areas referred to as [https://en.wikipedia.org/wiki/Complementarity-determining_region complementary-determining regions](CDRs), which are where the antigen makes <scene name='95/952713/Antigen_antibody_contact/2'>contact</scene> with the antibody on the Fab domain. Figure 2 depicts this as a surface representation given that the specific residues within the antigen-binding motif are unknown.
| + | The IgM-BCR contains areas referred to as [https://en.wikipedia.org/wiki/Complementarity-determining_region complementary-determining regions](CDRs), which are where the antigen makes <scene name='95/952713/Antigen_antibody_contact/2'>contact</scene> with the antibody on the Fab domain. Figure 2 depicts this as a surface representation given that the specific residues within the antigen-binding motif are unknown. |
| | | | |
| - | <div style="text-align: justify">Due to the poor resolution of the Fab region, specific side chain interactions between the heavy ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) and light (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) chains have not been determined. It is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. The <scene name='95/952713/Heavy-light_chain_interface/2'>heavy-light chain interface</scene> shows how the four heavy and light chain β-sandwiches fit together. The Fab region heavy chains attach to the Fc region heavy chains, before continuing down into the intracellular domain to interact with the <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> subunits. The light chains (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) however are only connected to the heavy chains ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) within the Fab region, thus have no contact with the <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> heterodimer.
| + | Due to the poor resolution of the Fab region, specific side chain interactions between the heavy ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) and light (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) chains have not been determined. It is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. The <scene name='95/952713/Heavy-light_chain_interface/2'>heavy-light chain interface</scene> shows how the four heavy and light chain β-sandwiches fit together. The Fab region heavy chains attach to the Fc region heavy chains, before continuing down into the intracellular domain to interact with the <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> subunits. The light chains (<b><span class="text-purple">A</span></b>/<b><span class="text-cyan">B</span></b>) however are only connected to the heavy chains ('''{{Font color|violet|A}}'''/<b><span class="text-blue">B</span></b>) within the Fab region, thus have no contact with the <b><span class="text-brown">Igα</span></b>/<b><span class="text-orange">Igβ</span></b> heterodimer. |
| | | | |
| | [[Image:IgM_Surface2.png|400 px|left|thumb|'''Figure 3. Surface Representation of IgM Antibody Binding Pocket.''' On one arm of the IgM antibody, the antigen makes contact with light chain A at the L1 and L3 complementary-determining regions. Furthermore, it makes contact with heavy chain A at the H1, H2, and H3 complementary-determining regions. The location of the complementary-determining regions were approximated using the structure of the VCR01 variable region and were visualized using PyMOL.]] | | [[Image:IgM_Surface2.png|400 px|left|thumb|'''Figure 3. Surface Representation of IgM Antibody Binding Pocket.''' On one arm of the IgM antibody, the antigen makes contact with light chain A at the L1 and L3 complementary-determining regions. Furthermore, it makes contact with heavy chain A at the H1, H2, and H3 complementary-determining regions. The location of the complementary-determining regions were approximated using the structure of the VCR01 variable region and were visualized using PyMOL.]] |
|
Introduction
The adaptive immune response possessed by vertebrate animals owes much of its function to B cells. These specialized immune cells produce antibodies and immunoglobulins (Ig), the membrane bound equivalent to antibodies. B cells can produce a variety of Ig compounds including IgG, IgA, IgE, IgD, and IgM. These antibodies and Ig compounds bind to specific compounds called antigens. When an IgM combines with a B cell receptor (BCR), it can then send a signal in the form of a conformational change through the B cell membrane to stimulate the production of more antibodies that recognize that antigen. [1]
The structure of the IgM BCR complex was determined by two research groups using Cryo EM. They also determined the structure of IgG. [2], [3]
Structure
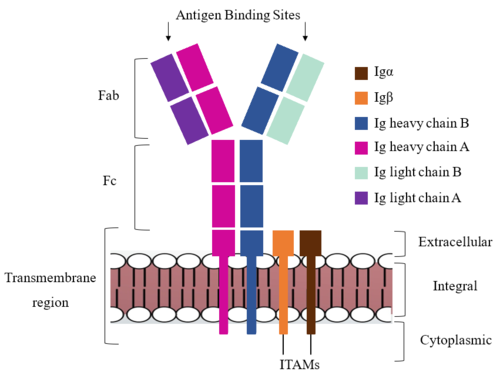 Figure 1. IgM BCR Structure Overview. Depiction of the IgM BCR expressed on the membrane of a B cell. Includes all major components including the α/β heterodimer, heavy and light chains, antigen binding sites, and the ITAM region for signal transduction. The IgM BCR consists of six separate chains (Figure 1) that make up the three main domains typically found in a general antibody structure. A depiction of the IgM shows two heavy and two light chains that together form the Fab region, or variable fragment at the top of the molecule where the antigen binding sites are located. The two heavy chains extend below the Fab region through the Fc region and eventually connect to the Igα/β heterodimer to form the transmembrane region which anchors the overall complex to the B cell. These regions are also labeled in Figure 1. The overall structure, expression, and function of the IgM BCR is strongly influenced by the transmembrane region in which Ig α/β interactions as a heterodimer influence cell surface expression, receptor assembly, and effective signal transduction. [4], [5]. In each domain, interactions between individual chains are important to understand the complex as a whole. All future 3D depictions showing these interactions ues the same PyMOL file (7xq8) and will be as in Figure 1.
Transmembrane Region
The IgM BCR is anchored to B-cell membranes through the which is broken up into both extracellular and integral domains which sit on top of or span through the membrane, respectively (Figure 1). The extracellualr region is primarily composed of β-sheetswhile the integral region is composed of α-helices. IgM BCR assembly requires dimerization of the Igα and Igβ subunits which embed within the B-cell membrane. [4] The dimerizes within the extracellular region with a . Additional dimerization occurs within the integral region via a hydrogen bond; the residues involved have not been confirmed. Although the mechanism of disulfide bridge formation is still unknown, via N-linked asparagine glycosyl groups (NAGs) in the extracellular region of both the Igα and and Igβ chains is hypothesized to facilitate this process. The NAG groups are believed to be essential for the recruitment of Chaperone proteins to optimize the folding process. [6] Past studies with human and viral proteins have shown that the presence of NAGs not only facilitate the rapid formation of disulfide bridges, but also ensure correct location. [7], [8] The recruited chaperone proteins will remain bound to the Igα and Igβ subunits until dimerization occurs. [5]
After Igα and Igβ dimerization, the transmembrane helices of the heavy chains can embed within the B-cell membrane and intertwine with the Igα and Igβ chains. [4] The side chains of this , made up by the alpha, beta, and heavy chains (A/B), are primarily hydrophobic; this allows for interactions with the hydrophobic tails in the phospholipid bilayer. The four helices (Figure 2) are intertwined and primarily held together through interactions between the ; however, a a few polar residues are included which allow for additional interactions with the polar residues on the Igα and Igβ chains. [5]
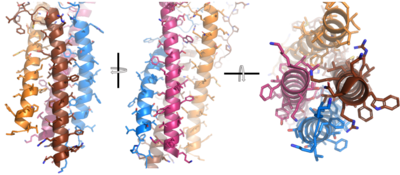 Figure 2. 4-pass integral helix. PyMOL image of the integral helices in IgM BCR (PDB:7xq8). The structure is shown rotated on the x and y axes to illustrate how the chains are intertwined. Side chains are shown as sticks. Brown=Ig alpha, orange=Ig beta, pink=heavy chain A, blue=heavy chain B. Within the transmembrane region, heavy chain A and heavy chain B associate (Figure 1) asymmetrically to facilitate intracellular signaling cascades. The allows them to pack together via Van der Waals contacts, but there are also prominent hydrogen bonds between each chain. More specifically, the hydroxyl group from Ser584 on heavy chain A donates a hydrogen bond to Ser584 and to Ser588 on heavy chain B. This creates a bifurcated hydrogen bond, essentially forming a “fork” between the two chains to help stabilize them and maintain the transmission of the signal once the cell is activated. Because transmembrane Ig molecules cannot efficiently initiate the signal cascade, they must associate with the Igα and Igβ chains within the BCR. [2]
Furthermore, both the Igα and Igβ chains have cytoplasmic tails that extend into the B cell (Figure 1). Each of these tails contain an ITAM region to facilitate signal transduction (Figure 4). [3] The structures of the ITAM regions have not yet been determined.
Fc Region
The constant region of IgM is made up of the two . These heavy chains form a bridge connecting the FAB region or variable region to the transmembrane region (Figure 1). They also act as a wire that the variable region can send a signal through to the transmembrane region as a mechanical change.
help hold the heavy chains and Igα/Igβ chains together in the extracellular portion of the transmembrane region.
Because a conformational change occurs throughout the entirety of the IgM-BCR complex, the Fc region must be able to tolerate the contortion of the molecule as the antigen binds. In constant region two, which is located at the start of the Fc region, heavy chain A and heavy chain B make a to stabilize the IgM-BCR and drive downstream signaling.
To maximize the Fc region’s signal transduction efficiency and Van der Waals contacts, constant region two of heavy chain A makes an asymmetrical association with constant region three of heavy chain B to create a . More specifically, Arg243 and Arg251 residues from heavy chain A donate three hydrogen bonds to Leu433, Thr431, and Asp376 residues on heavy chain B. Furthermore, Leu313 of heavy chain A accepts a hydrogen bond from Thr429 on heavy chain B. [3]
Fab Region
The Fab region of the antibody is where antigen recognition occurs upon binding (Figure 1). On each arm is one heavy (A/B) and one light (A/B) chain, both containing domains identical to their respective counterparts. Repeats of β-sandwiches form the constant and variable domains within the Fab region as antigen recognition occurs at the variable domain while the constant domain connects it to the rest of the IgM complex. Because the Fab region of IgM is poorly resolved, a structural analysis of an HIV neutralizing antibody called VCR01 was performed to approximate where an antigen would bind to at the . [9]
The IgM-BCR contains areas referred to as complementary-determining regions(CDRs), which are where the antigen makes with the antibody on the Fab domain. Figure 2 depicts this as a surface representation given that the specific residues within the antigen-binding motif are unknown.
Due to the poor resolution of the Fab region, specific side chain interactions between the heavy (A/B) and light (A/B) chains have not been determined. It is estimated that each β-sandwich contains one disulfide bridge with additional hydrogen bonds. The shows how the four heavy and light chain β-sandwiches fit together. The Fab region heavy chains attach to the Fc region heavy chains, before continuing down into the intracellular domain to interact with the Igα/Igβ subunits. The light chains (A/B) however are only connected to the heavy chains (A/B) within the Fab region, thus have no contact with the Igα/Igβ heterodimer.
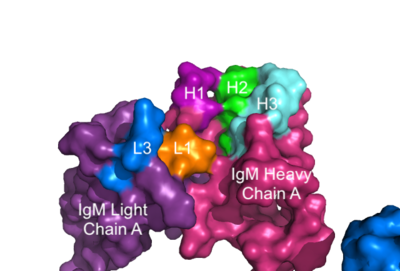 Figure 3. Surface Representation of IgM Antibody Binding Pocket. On one arm of the IgM antibody, the antigen makes contact with light chain A at the L1 and L3 complementary-determining regions. Furthermore, it makes contact with heavy chain A at the H1, H2, and H3 complementary-determining regions. The location of the complementary-determining regions were approximated using the structure of the VCR01 variable region and were visualized using PyMOL. Signal Transduction
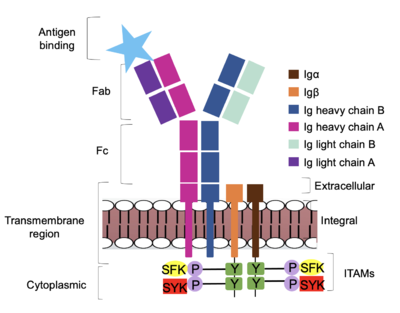 Figure 4. IgM Antibody Signal Transduction following Antigen Binding. At the end of the intracellular Igα and Igβ helices are their cytoplasmic tails, and on each tail are tyrosine residues that are phosphorylated by one of two tyrosine kinase enzymes: Splenic-tyrosine kinase and Src family kinase. While the specific tyrosine residues are unknown in the mechanism, it is understood that their phosphorylation activates the B cell by triggering downstream intracellular signaling. The diagram in Figure 4 depicts the initial process of B cell activation by the antigen binding to the antibody at the Fab region. The underlying mechanism for signal transduction is unknown but it is speculated to operate under what is known as the conserved assembly mechanism. [3] This means that upon antigen binding, BCRs on the surface of the cell begin to cluster to cause the phosphorylation of the immunoreceptor tyrosine-based activation motifs located in Igα and Igβ. In its “off” state, the constant region of heavy chain B overlaps the extracellular components of Igα and Igβ. As the antigen binds, it induces a conformational change to release the overlap and allow for clustering about the BCR. Now, in its “on” state the phosphorylation of the ITAM region (observed in figure 4 as conserved phosphorylated tyrosine residues) within the intracellular tails of Igα and Igβ drives downstream kinase activity to continue to process of signal cascading.
|




