We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | ==Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)== | + | ==Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) <ref>Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. |
| + | https://nsuworks.nova.edu/protein_modeling_reports/7</ref>== | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
| Line 9: | Line 10: | ||
== Introduction == | == Introduction == | ||
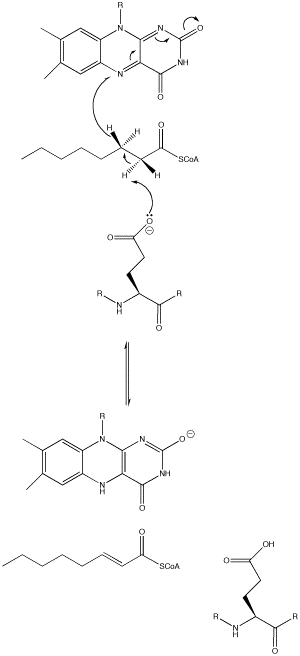
| - | An important enzyme in β-oxidation is Acyl-CoA Dehydrogenase, which abstracts a hydrogen atom from its fatty acyl-CoA substrate and inserts it on FAD, an electron carrier. With FAD also removing a fatty acyl-CoA hydrogen, FAD is reduced to FADH2, which is utilized in the electron transport chain to ultimately produce ATP, forming a double bond on the acyl-CoA chain. In Medium Acyl-CoA Dehydrogenase Deficiency (MCADD), mutations in the ACADM (Acyl-CoA Dehydrogenase Medium-Chain) gene, the only gene that causes MCADD <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>, render less functional MCADs. Since MCADD is the most common defect in the pathway of β-oxidation, and MCAD (medium-chain acyl-CoA dehydrogenase) is needed to metabolize medium-chain fatty acids, a deficiency of this protein has effects ranging from hypoglycemia and lethargy, and damage to the brain and liver due to a buildup of fatty tissue <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>. Understanding of the mutations that caused the disease was sought; amino acid mutations that overlapped across the studies researched and were able to be visualized in the Human WT MCAD (PDB ID: 1T9G) were recorded and analyzed for their effects on the protein (i.e., helix-helix interactions, H-bonding to ligand) and how it could contribute to MCAD; these mutations are listed in the colored table to the | + | An important enzyme in β-oxidation is Acyl-CoA Dehydrogenase, which abstracts a hydrogen atom from its fatty acyl-CoA substrate and inserts it on FAD, an electron carrier. With FAD also removing a fatty acyl-CoA hydrogen, FAD is reduced to FADH2, which is utilized in the electron transport chain to ultimately produce ATP, forming a double bond on the acyl-CoA chain. In Medium Acyl-CoA Dehydrogenase Deficiency (MCADD), mutations in the ACADM (Acyl-CoA Dehydrogenase Medium-Chain) gene, the only gene that causes MCADD <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>, render less functional MCADs. Since MCADD is the most common defect in the pathway of β-oxidation, and MCAD (medium-chain acyl-CoA dehydrogenase) is needed to metabolize medium-chain fatty acids, a deficiency of this protein has effects ranging from hypoglycemia and lethargy, and damage to the brain and liver due to a buildup of fatty tissue <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>. Understanding of the mutations that caused the disease was sought; amino acid mutations that overlapped across the studies researched and were able to be visualized in the Human WT MCAD (PDB ID: 1T9G) were recorded and analyzed for their effects on the protein (i.e., helix-helix interactions, H-bonding to ligand) and how it could contribute to MCAD; these mutations are listed in the colored table to the below. |
| + | |||
| + | [[Image:AcylCoAdehydrogenase.png]] | ||
== Materials & Methods == | == Materials & Methods == | ||
Revision as of 19:21, 21 May 2023
Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) [1]
| |||||||||||
References
- ↑ Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7