We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
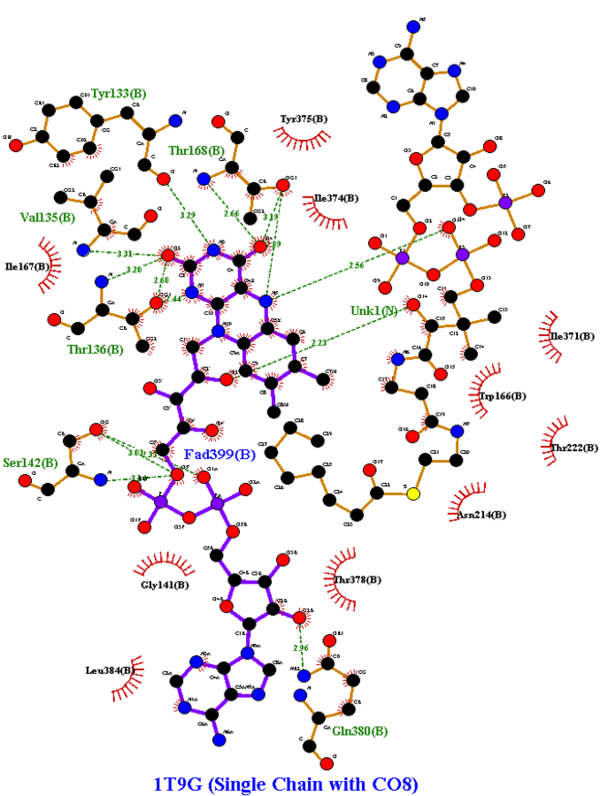
LigPlot+ was used to analyze and determine the active site of the modified 1T9G protein: | LigPlot+ was used to analyze and determine the active site of the modified 1T9G protein: | ||
| - | [[Image:Omar Saleh LigPlot Figure.png|600px|thumb|center|'''Figure 2''' | + | [[Image:Omar Saleh LigPlot Figure.png|600px|thumb|center|'''Figure 2:''' LigPlot+ results of the computerized-specific 1T9G, with hydrogen bonds shown in olive green, hydrophobic interactions shown in brick red, and UNK as CO8; clarifies Model C of Figure 3.]] |
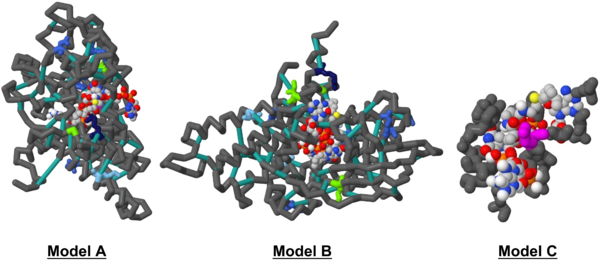
| - | The ultimate result of this project (and course) was to create a 3D model in Jmol, shown in detail below | + | The ultimate result of this project (and course) was to create a 3D model in Jmol, shown in detail below: |
[[Image:Omar_Saleh_Jmol_Figures.png|600px|thumb|center|'''Figure 3:''' Models A & B are identical, differing only in rotation. For the color scheme of both, the backbone was colored in “dimgray”, the ligands in the common atom identity color scheme “CPK” with “lightgrey” carbons, and the struts “lightseagreen”; amnio acid colors are specified in Table 1 below. Model C focuses on the active site in greater detail using the LigPlot+ result data.]] | [[Image:Omar_Saleh_Jmol_Figures.png|600px|thumb|center|'''Figure 3:''' Models A & B are identical, differing only in rotation. For the color scheme of both, the backbone was colored in “dimgray”, the ligands in the common atom identity color scheme “CPK” with “lightgrey” carbons, and the struts “lightseagreen”; amnio acid colors are specified in Table 1 below. Model C focuses on the active site in greater detail using the LigPlot+ result data.]] | ||
Revision as of 22:38, 21 May 2023
Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) [1]
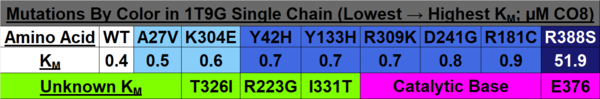
| |||||||||||
References
- ↑ Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7
- ↑ https://medlineplus.gov/genetics/condition/medium-chain-acyl-coa-dehydrogenase-deficiency/
- ↑ Bach, R. D., Thorpe, C., & Dmitrenko, O. (n.d.). Synergy Between H-Bonding Interactions and Its Role in the Enzyme-Catalyzed a-Proton Abstraction. DFT Studies On the Acyl-CoA Dehydrogenase Model Systems. University of Delaware. https://www1.udel.edu/chem/bach/pages/CCE8corr.html
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Toogood, H. S., van Thiel, A., Basran, J., Sutcliffe, M. J., Scrutton, N. S., & Leys, D. (2004). Extensive domain motion and electron transfer in the human electron transferring flavoprotein·medium chain acyl-COA dehydrogenase complex. Journal of Biological Chemistry, 279(31), 32904–32912. https://doi.org/10.1074/jbc.m404884200
- ↑ Maier, E. M., Gersting, S. W., Kemter, K. F., Jank, J. M., Reindl, M., Messing, D. D., Truger, M. S., Sommerhoff, C. P., & Muntau, A. C. (2009). Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. Human molecular genetics, 18(9), 1612–1623. https://doi.org/10.1093/hmg/ddp079
- ↑ McAndrew, R. P., Wang, Y., Mohsen, A. W., He, M., Vockley, J., & Kim, J. J. (2008). Structural basis for substrate fatty acyl chain specificity: crystal structure of human very-long-chain acyl-CoA dehydrogenase. The Journal of biological chemistry, 283(14), 9435–9443. https://doi.org/10.1074/jbc.M709135200
- ↑ Tucci, S., Wagner, C., Grünert, S. C., Matysiak, U., Weinhold, N., Klein, J., Porta, F., Spada, M., Bordugo, A., Rodella, G., Furlan, F., Sajeva, A., Menni, F., & Spiekerkoetter, U. (2021). Genotype and residual enzyme activity in medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Are predictions possible? Journal of inherited metabolic disease, 44(4), 916–925. https://doi.org/10.1002/jimd.12368