We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
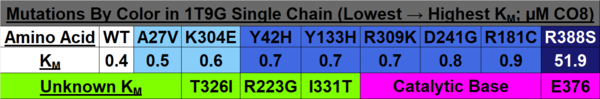
Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
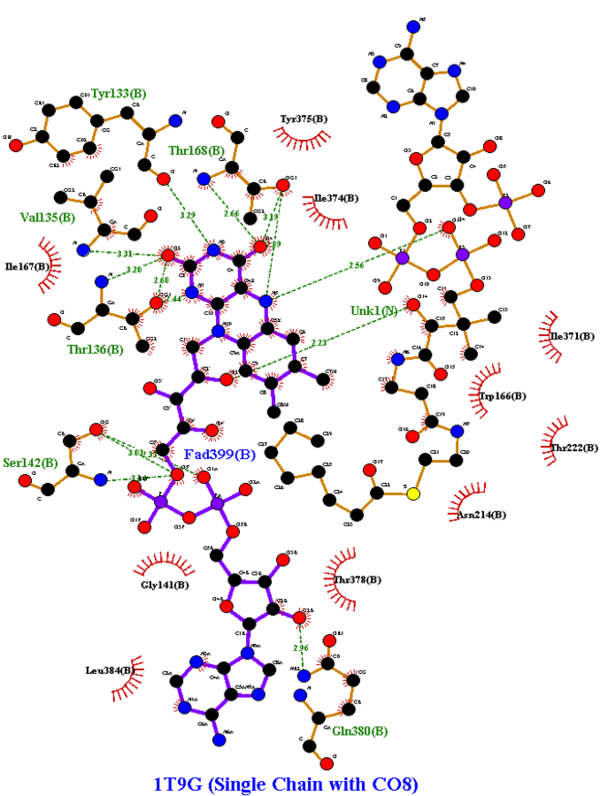
An important enzyme in β-oxidation is Acyl-CoA Dehydrogenase, which abstracts a hydrogen atom from its fatty acyl-CoA substrate and inserts it on FAD, an electron carrier. With FAD also removing a fatty acyl-CoA hydrogen, FAD is reduced to FADH2, which is utilized in the electron transport chain to ultimately produce ATP, forming a double bond on the acyl-CoA chain. The biochemical mechanism is shown in the image below<ref>Bach, R. D., Thorpe, C., & Dmitrenko, O. (n.d.). Synergy Between H-Bonding Interactions and Its Role in the Enzyme-Catalyzed a-Proton Abstraction. DFT Studies On the Acyl-CoA Dehydrogenase Model Systems. University of Delaware. https://www1.udel.edu/chem/bach/pages/CCE8corr.html</ref>. In Medium Acyl-CoA Dehydrogenase Deficiency (MCADD), mutations in the ACADM (Acyl-CoA Dehydrogenase Medium-Chain) gene, the only gene that causes MCADD <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>, render less functional MCADs. Since MCADD is the most common defect in the pathway of β-oxidation, and MCAD (medium-chain acyl-CoA dehydrogenase) is needed to metabolize medium-chain fatty acids, a deficiency of this protein has effects ranging from hypoglycemia and lethargy, and damage to the brain and liver due to a buildup of fatty tissue <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>. Understanding of the mutations that caused the disease was sought; amino acid mutations that overlapped across the studies researched and were able to be visualized in the Human WT MCAD (PDB ID: 1T9G) were recorded and analyzed for their effects on the protein (i.e., helix-helix interactions, H-bonding to ligand) and how it could contribute to MCAD; these mutations are listed in the colored table to the below. | An important enzyme in β-oxidation is Acyl-CoA Dehydrogenase, which abstracts a hydrogen atom from its fatty acyl-CoA substrate and inserts it on FAD, an electron carrier. With FAD also removing a fatty acyl-CoA hydrogen, FAD is reduced to FADH2, which is utilized in the electron transport chain to ultimately produce ATP, forming a double bond on the acyl-CoA chain. The biochemical mechanism is shown in the image below<ref>Bach, R. D., Thorpe, C., & Dmitrenko, O. (n.d.). Synergy Between H-Bonding Interactions and Its Role in the Enzyme-Catalyzed a-Proton Abstraction. DFT Studies On the Acyl-CoA Dehydrogenase Model Systems. University of Delaware. https://www1.udel.edu/chem/bach/pages/CCE8corr.html</ref>. In Medium Acyl-CoA Dehydrogenase Deficiency (MCADD), mutations in the ACADM (Acyl-CoA Dehydrogenase Medium-Chain) gene, the only gene that causes MCADD <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>, render less functional MCADs. Since MCADD is the most common defect in the pathway of β-oxidation, and MCAD (medium-chain acyl-CoA dehydrogenase) is needed to metabolize medium-chain fatty acids, a deficiency of this protein has effects ranging from hypoglycemia and lethargy, and damage to the brain and liver due to a buildup of fatty tissue <ref>Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090</ref>. Understanding of the mutations that caused the disease was sought; amino acid mutations that overlapped across the studies researched and were able to be visualized in the Human WT MCAD (PDB ID: 1T9G) were recorded and analyzed for their effects on the protein (i.e., helix-helix interactions, H-bonding to ligand) and how it could contribute to MCAD; these mutations are listed in the colored table to the below. | ||
| - | https://www1.udel.edu/chem/bach/pages/Image1.gif | + | [[Image:https://www1.udel.edu/chem/bach/pages/Image1.gif|thumb|'''Figure 1:''' Brief summary of the MCAD mechanism.]] |
== Materials & Methods == | == Materials & Methods == | ||
Revision as of 22:40, 21 May 2023
Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) [1]
| |||||||||||
References
- ↑ Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7
- ↑ https://medlineplus.gov/genetics/condition/medium-chain-acyl-coa-dehydrogenase-deficiency/
- ↑ Bach, R. D., Thorpe, C., & Dmitrenko, O. (n.d.). Synergy Between H-Bonding Interactions and Its Role in the Enzyme-Catalyzed a-Proton Abstraction. DFT Studies On the Acyl-CoA Dehydrogenase Model Systems. University of Delaware. https://www1.udel.edu/chem/bach/pages/CCE8corr.html
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Toogood, H. S., van Thiel, A., Basran, J., Sutcliffe, M. J., Scrutton, N. S., & Leys, D. (2004). Extensive domain motion and electron transfer in the human electron transferring flavoprotein·medium chain acyl-COA dehydrogenase complex. Journal of Biological Chemistry, 279(31), 32904–32912. https://doi.org/10.1074/jbc.m404884200
- ↑ Maier, E. M., Gersting, S. W., Kemter, K. F., Jank, J. M., Reindl, M., Messing, D. D., Truger, M. S., Sommerhoff, C. P., & Muntau, A. C. (2009). Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. Human molecular genetics, 18(9), 1612–1623. https://doi.org/10.1093/hmg/ddp079
- ↑ McAndrew, R. P., Wang, Y., Mohsen, A. W., He, M., Vockley, J., & Kim, J. J. (2008). Structural basis for substrate fatty acyl chain specificity: crystal structure of human very-long-chain acyl-CoA dehydrogenase. The Journal of biological chemistry, 283(14), 9435–9443. https://doi.org/10.1074/jbc.M709135200
- ↑ Tucci, S., Wagner, C., Grünert, S. C., Matysiak, U., Weinhold, N., Klein, J., Porta, F., Spada, M., Bordugo, A., Rodella, G., Furlan, F., Sajeva, A., Menni, F., & Spiekerkoetter, U. (2021). Genotype and residual enzyme activity in medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Are predictions possible? Journal of inherited metabolic disease, 44(4), 916–925. https://doi.org/10.1002/jimd.12368