We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Investigating the Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)
From Proteopedia
(Difference between revisions)
| Line 37: | Line 37: | ||
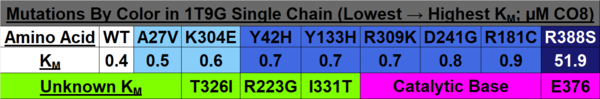
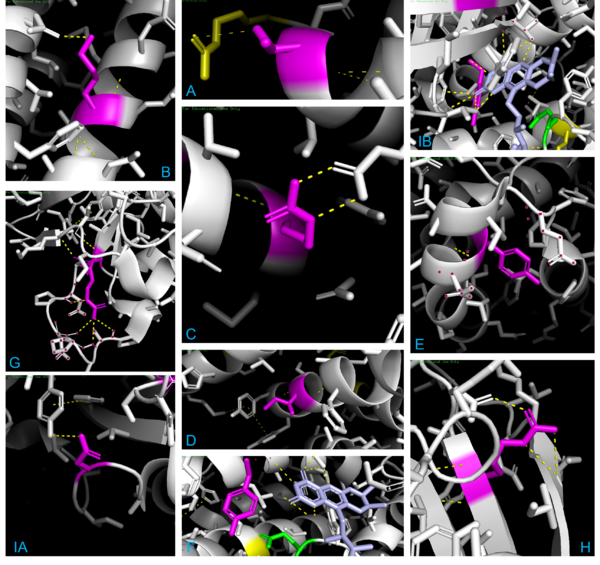
When observing 1T9G in PyMOL (see Figure 4 below), there are 3 arginine residues (R256, R324, & R388) that interact with CO8, so R388S would greatly lessen ligand interaction, explaining the high KM outlier. T326 interacted with R324, so although the KM value is unknown, it is predicted that the KM for T326I would lie within a high range, as it was found to lead to aggregation and thus a loss of function. K304 & R309 impact helix-helix stability via interactions with Q342 & E85, respectively; thus, mutations would destabilize them, explaining R309K’s higher KM. I331T would destabilize two α-helices by disrupting a series of π-interactions; while its KM is unknown, it is predicted that it lies within the intermediate/high range described previously, as it led to a complete loss of protein function due to aggregation. Y133 maps to the β-sheet domain, directly H-bonding with FAD along with the residues T136 & T168; Y133H might interact with E376 and impair helix-helix stability, explaining its intermediate KM. D241 stabilizes a β-sheet whose end residue bonds to FAD, explaining its high KM. R223G would unlink β-sheet interactions; while its KM is unknown, it was predicted to be within the higher range as it was shown to have a complete loss of function due to aggregation, just like T326I & I331T. R181, while far from the active site, makes four bonds with other amino acids, thus explaining its second-highest KM when mutated. A27V is mostly unaffected regarding both size & charge, explaining its KM similarity to the WT. Y42H might cause an interaction with nearby E41 & E47, explaining its lower intermediate KM. The 3D model helps visualize these mutations and their effects. | When observing 1T9G in PyMOL (see Figure 4 below), there are 3 arginine residues (R256, R324, & R388) that interact with CO8, so R388S would greatly lessen ligand interaction, explaining the high KM outlier. T326 interacted with R324, so although the KM value is unknown, it is predicted that the KM for T326I would lie within a high range, as it was found to lead to aggregation and thus a loss of function. K304 & R309 impact helix-helix stability via interactions with Q342 & E85, respectively; thus, mutations would destabilize them, explaining R309K’s higher KM. I331T would destabilize two α-helices by disrupting a series of π-interactions; while its KM is unknown, it is predicted that it lies within the intermediate/high range described previously, as it led to a complete loss of protein function due to aggregation. Y133 maps to the β-sheet domain, directly H-bonding with FAD along with the residues T136 & T168; Y133H might interact with E376 and impair helix-helix stability, explaining its intermediate KM. D241 stabilizes a β-sheet whose end residue bonds to FAD, explaining its high KM. R223G would unlink β-sheet interactions; while its KM is unknown, it was predicted to be within the higher range as it was shown to have a complete loss of function due to aggregation, just like T326I & I331T. R181, while far from the active site, makes four bonds with other amino acids, thus explaining its second-highest KM when mutated. A27V is mostly unaffected regarding both size & charge, explaining its KM similarity to the WT. Y42H might cause an interaction with nearby E41 & E47, explaining its lower intermediate KM. The 3D model helps visualize these mutations and their effects. | ||
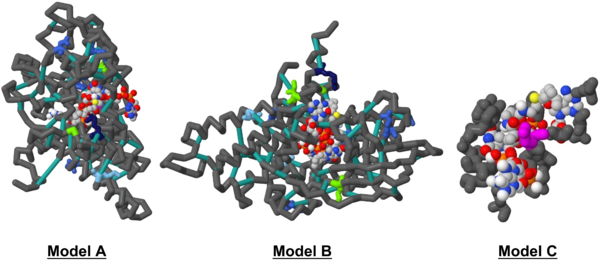
| - | + | [[Image:Omar Saleh PyMOL Figures.png|600px|thumb|center|'''Figure 4:''' Interactions for the locations T326 (A), K304 (B), R309 (C), I331 (D), Y42 (E), Y133 (F), R181 (G), R223 (H), & D241 (IA) and its β-sheet tip (IB). For each, the focused residues are magenta, dashed bonds yellow, catalytic E376 green, key arginine residues (R256, R324, & R388) in yellow, backbone in white, and specific residues selected/dotted to clarify bonding.]] | |
== Applications == | == Applications == | ||
Revision as of 22:44, 21 May 2023
Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD) [1]
| |||||||||||
References
- ↑ Saleh, Omar E.; Khatiwala, Rhea; and Ignatius, Jeremy, "Investigating The Mechanisms of Active Site Mutations to the 1T9G WT MCAD Protein to Better Understand Medium Chain Acyl-CoA Dehydrogenase Deficiency (MCADD)" (2022). Protein Modeling Reports. 7. https://nsuworks.nova.edu/protein_modeling_reports/7
- ↑ https://medlineplus.gov/genetics/condition/medium-chain-acyl-coa-dehydrogenase-deficiency/
- ↑ Bach, R. D., Thorpe, C., & Dmitrenko, O. (n.d.). Synergy Between H-Bonding Interactions and Its Role in the Enzyme-Catalyzed a-Proton Abstraction. DFT Studies On the Acyl-CoA Dehydrogenase Model Systems. University of Delaware. https://www1.udel.edu/chem/bach/pages/CCE8corr.html
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Drendel, H. M., Pike, J. E., Schumacher, K., Ouyang, K., Wang, J., Stuy, M., Dlouhy, S., & Bai, S. (2015). Intermediate MCAD Deficiency Associated with a Novel Mutation of the ACADM Gene: c.1052C>T. Case reports in genetics, 2015, 532090. https://doi.org/10.1155/2015/532090
- ↑ Toogood, H. S., van Thiel, A., Basran, J., Sutcliffe, M. J., Scrutton, N. S., & Leys, D. (2004). Extensive domain motion and electron transfer in the human electron transferring flavoprotein·medium chain acyl-COA dehydrogenase complex. Journal of Biological Chemistry, 279(31), 32904–32912. https://doi.org/10.1074/jbc.m404884200
- ↑ Maier, E. M., Gersting, S. W., Kemter, K. F., Jank, J. M., Reindl, M., Messing, D. D., Truger, M. S., Sommerhoff, C. P., & Muntau, A. C. (2009). Protein misfolding is the molecular mechanism underlying MCADD identified in newborn screening. Human molecular genetics, 18(9), 1612–1623. https://doi.org/10.1093/hmg/ddp079
- ↑ McAndrew, R. P., Wang, Y., Mohsen, A. W., He, M., Vockley, J., & Kim, J. J. (2008). Structural basis for substrate fatty acyl chain specificity: crystal structure of human very-long-chain acyl-CoA dehydrogenase. The Journal of biological chemistry, 283(14), 9435–9443. https://doi.org/10.1074/jbc.M709135200
- ↑ Tucci, S., Wagner, C., Grünert, S. C., Matysiak, U., Weinhold, N., Klein, J., Porta, F., Spada, M., Bordugo, A., Rodella, G., Furlan, F., Sajeva, A., Menni, F., & Spiekerkoetter, U. (2021). Genotype and residual enzyme activity in medium-chain acyl-CoA dehydrogenase (MCAD) deficiency: Are predictions possible? Journal of inherited metabolic disease, 44(4), 916–925. https://doi.org/10.1002/jimd.12368