User:Francielle Aguiar Gomes/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
==Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum''== | ==Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum''== | ||
| - | <StructureSection load='7EQD' size='340' side='right' caption='Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum''' scene=''> | ||
| + | <StructureSection load='7EQD' size='340' side='right' caption='Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum''' scene=''> | ||
== Introduction == | == Introduction == | ||
| - | Rhodospirillum ''(Rsp.) rubrum'' is an anoxygenic phototrophic purple bacterium with a long history as a model for the study of bacterial photosynthesis and related metabolic processes. It is unique among purple bacteria by producing both rhodoquinone (RQ) and ubiquinone (UQ)1 as electron carriers and bacteriochlorophyll (BChl) a esterified at the propionic acid side chain by geranylgeraniol (abbreviated as | + | Rhodospirillum ''(Rsp.) rubrum'' is an anoxygenic phototrophic purple bacterium with a long history as a model for the study of bacterial photosynthesis and related metabolic processes. It is unique among purple bacteria by producing both rhodoquinone (RQ) and ubiquinone (UQ)1 as electron carriers and bacteriochlorophyll (BChl) a esterified at the propionic acid side chain by geranylgeraniol (abbreviated as BChlaG) rather than phytol. |
| - | (RC) cytochrome (Cyt) c subunit present in many purple bacteria; thus, ''Rsp. rubrum'' is one of the simplest phototrophic bacteria known, in terms of its photosynthetic light reactions. Because the entire Rsp. rubrum LH1 complex and a stable B820 LH1-subunit can be reconstituted using the αβ-polypeptides | + | The light-harvesting complexes (LHC) of photosynthetic purple sulfur and non-sulfur bacteria are responsible for the highly efficient collection and transfer of light energy to the photosynthetic reaction centres. This results in an initial separation of charge in the reaction centre (RC) and ultimately conversion of the light energy into a chemically useful form <ref>10.1002/j.1460-2075.1995.tb07041.x</ref>. |
| - | and pigment molecules,3−5 both complexes have been intensively studied as models of the bacterial antenna apparatus6 and as such have provided a wealth of information on mechanisms of light energy acquisition, pigment−protein interactions, and assembly of multicomponent complexes. <ref>10.1021/acs.biochem.1c00360</ref> | + | ''Rsp. rubrum'' has a single pair of αβ-polypeptides in its core light-harvesting (LH1) complex and lacks both the peripheral light-harvesting (LH2) complex and reaction center (RC) cytochrome (Cyt) c subunit present in many purple bacteria; thus, ''Rsp. rubrum'' is one of the simplest phototrophic bacteria known, in terms of its photosynthetic light reactions. Because the entire Rsp. rubrum LH1 complex and a stable B820 LH1-subunit can be reconstituted using the αβ-polypeptides and pigment molecules,3−5 both complexes have been intensively studied as models of the bacterial antenna apparatus6 and as such have provided a wealth of information on mechanisms of light energy acquisition, pigment−protein interactions, and assembly of multicomponent complexes. <ref>10.1021/acs.biochem.1c00360</ref> |
| - | == | + | == Inicial Structures == |
| - | Structures of both purified LH1 and the RC-associated core complex (LH1-RC) of ''Rsp. rubrum'' have not been obtained at high resolution, and no RC atomic structure | + | Structures of both purified LH1 and the RC-associated core complex (LH1-RC) of ''Rsp. rubrum'' have not been obtained at high resolution, and no RC atomic structure was known. The 8.5 Å resolution projection of ''R. rubrum'' LHCl represents the first glimpse of the structural architecture of the fundamental building block of the photosynthetic membrane in purple BChla-containing bacteria. The crystals diffract beyond 8 Å and the projection map was calculated to 8.5 A. The projection map shows 16 subunits in a 116 Å diameter ring with a 68 Å hole in the center. |
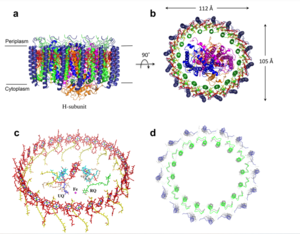
| - | [[Image:Structure.png|300px|left|thumb| Structure overview of the Rsp. rubrum LH1-RC complex. (a) Side view of the LH1-RC parallel to the membrane plane. (b) Top view of the LH1-RC from the periplasmic side of the membrane. (c) Tilted view of the cofactor arrangement. (d) Superposition of Cα carbons of the LH1 αβpolypeptides between Rsp. rubrum and Tch. tepidum (gray, PDB: 5Y5S). Color scheme: LH1-α, green; LH1-β, slate-blue; L-subunit, magenta; Msubunit, blue; BChl aG in LH1 and special pair, red sticks; Accessory BChl aG, cyan sticks; BPhe aG, light-pink sticks; Spirilloxanthin, yellow sticks; UQ10, blue sticks; RQ-10, green sticks; Fe, magenta ball. Phospholipids and detergents are omitted for clarity]] | + | [[Image:Structure.png|300px|left|thumb| '''Fig. 1.''' Structure overview of the Rsp. rubrum LH1-RC complex. (a) Side view of the LH1-RC parallel to the membrane plane. (b) Top view of the LH1-RC from the periplasmic side of the membrane. (c) Tilted view of the cofactor arrangement. (d) Superposition of Cα carbons of the LH1 αβpolypeptides between Rsp. rubrum and Tch. tepidum (gray, PDB: 5Y5S). Color scheme: LH1-α, green; LH1-β, slate-blue; L-subunit, magenta; Msubunit, blue; BChl aG in LH1 and special pair, red sticks; Accessory BChl aG, cyan sticks; BPhe aG, light-pink sticks; Spirilloxanthin, yellow sticks; UQ10, blue sticks; RQ-10, green sticks; Fe, magenta ball. Phospholipids and detergents are omitted for clarity]] |
== Cryo-EM == | == Cryo-EM == | ||
| - | Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied to samples cooled to cryogenic temperatures. For biological samples, structure is preserved by embedding in a glassy ice environment. An aqueous sample is applied to a mesh grid and frozen by immersion in liquid ethane or a mixture of liquid ethane and propane <ref>10.1017/S1431927608080781</ref>. This technique has advanced dramatically to become a viable tool for high-resolution structural biology research. The ultimate outcome of a cryoEM study is an atomic model of a macromolecule or its complex with interacting partners. Recent advances in direct electron detectors as well as reconstruction single particle algorithms have led to the determination of the structure of macromolecular complexes ranging from 2 to 5 Å resolution. At these resolutions, also known as “near atomic” resolution, it is possible to infer all-atom structures de novo. | + | '''Cryogenic electron microscopy''' (cryo-EM) is a cryomicroscopy technique applied to samples cooled to cryogenic temperatures. For biological samples, structure is preserved by embedding in a glassy ice environment. An aqueous sample is applied to a mesh grid and frozen by immersion in liquid ethane or a mixture of liquid ethane and propane <ref>10.1017/S1431927608080781</ref>. This technique has advanced dramatically to become a viable tool for high-resolution structural biology research. The ultimate outcome of a cryoEM study is an atomic model of a macromolecule or its complex with interacting partners. Recent advances in direct electron detectors as well as reconstruction single particle algorithms have led to the determination of the structure of macromolecular complexes ranging from 2 to 5 Å resolution. At these resolutions, also known as “near atomic” resolution, it is possible to infer all-atom structures de novo. |
The first step in cryoEM structure determination is de novo structure determination, where an initial model can be built, given only one sequence and a reconstruction, when no other limited structural information is known. In the second stage, the model is optimized, where a wide range of class of methods for improving the fit of a model to the data and improving the geometry of a model. Finally, tools for model validation are described, in attempt to quantify the overall accuracy of a model given a reconstruction. | The first step in cryoEM structure determination is de novo structure determination, where an initial model can be built, given only one sequence and a reconstruction, when no other limited structural information is known. In the second stage, the model is optimized, where a wide range of class of methods for improving the fit of a model to the data and improving the geometry of a model. Finally, tools for model validation are described, in attempt to quantify the overall accuracy of a model given a reconstruction. | ||
| - | [[Image: | + | [[Image:Structure1.png]] |
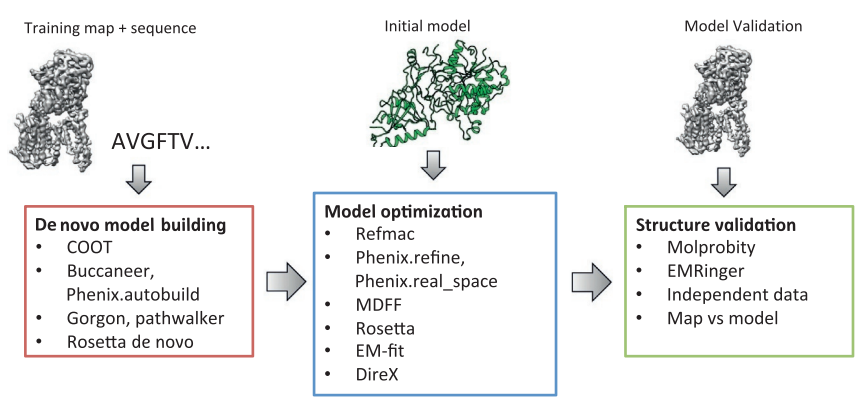
| + | An overview of three steps of atomic model determination from near-atomic resolution data. (Left) De novo building methods take primary sequence and map, and automatically produce a backbone model with sequence registered, identifying which regions in the map correspond to particular sequences. (Center) Model optimization takes an initial model—either produced from de novo building, or from a highresolution homologue—and optimizes the coordinates to better agree with the map, as well as adopt more physically realistic geometry. (Right) Model validation aims to assess—both globally and locally—the accuracy of a model, given experimental data. Such tools are useful not only for assessing overall accuracy but also for tuning parameters of optimization<ref>10.1016/bs.mie.2016.06.003</ref>. | ||
== Structure of Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum'' == | == Structure of Photosynthetic LH1-RC Super-complex of ''Rhodospirillum rubrum'' == | ||
Revision as of 19:06, 8 June 2023
Photosynthetic LH1-RC Super-complex of Rhodospirillum rubrum
| |||||||||||