User:Daniel Key Takemoto/Sandbox 1
From Proteopedia
m |
|||
| Line 28: | Line 28: | ||
It's been shown that, when the FMRP is in the nucleus, the Agenet domain allow the FMRP to interact with the chromatin and regulate DNA damage response. | It's been shown that, when the FMRP is in the nucleus, the Agenet domain allow the FMRP to interact with the chromatin and regulate DNA damage response. | ||
| - | And has also been demonstrated that the Agenet domains bind to methylated lysine, in support to the histone binding function of the FMRP, due to its aromatic cage in the structure; specifically, the Agenet motif 2 binds to trimethylated lysine. The <scene name='96/969643/Aminoacid_residues/1'>Aromatic aminoacid residues</scene> highlited in red are part of the Agenet 1 and the ones highlited in blue are part of the Agenet 2 domain related to the recognition of methylated lysine as they all have aromatic cages with the potential for this binding. | + | And has also been demonstrated that the Agenet domains bind to methylated lysine, in support to the histone binding function of the FMRP, due to its aromatic cage in the structure; specifically, the Agenet motif 2 binds to trimethylated lysine. The <scene name='96/969643/Aminoacid_residues/1'>Aromatic aminoacid residues</scene> highlited in red are part of the Agenet 1 and the ones highlited in blue are part of the Agenet 2 domain related to the '''recognition of methylated lysine''' as they all have '''aromatic cages''' with the potential for this binding. |
<ref>MYRICK, L. K. et al. Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human Molecular Genetics, v. 24, n. 6, p. 1733–1740, 20 nov. 2014.[https://doi.org/10.1093/hmg/ddu586]</ref>. | <ref>MYRICK, L. K. et al. Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human Molecular Genetics, v. 24, n. 6, p. 1733–1740, 20 nov. 2014.[https://doi.org/10.1093/hmg/ddu586]</ref>. | ||
The K-homology domain (KH domain) folding is similar to other RBPs domains, which play an important role in RNA binding and protein-protein interactions. | The K-homology domain (KH domain) folding is similar to other RBPs domains, which play an important role in RNA binding and protein-protein interactions. | ||
| - | Here it is the <scene name='96/969643/Kh0_motif_assym_new/1'>KH0 motif</scene> highlighted in red. Many RNA-binding proteins contain the KH motif, a conserved RNA-binding domain, but different from the KH1 and KH2 motifs, which contain a GXXG canonical motif, that will be explained in further details in the next topic, instead this one has an A-K-E-A. It is most likely involved in RNA binding and regulation, as seen in other proteins that also contain KH motifs. To give a more thorough response addressing the precise role played by the KH theme in FMRP, additional details would be needed. | + | Here it is the <scene name='96/969643/Kh0_motif_assym_new/1'>KH0 motif</scene> highlighted in red. Many RNA-binding proteins contain the KH motif, a conserved RNA-binding domain, but different from the KH1 and KH2 motifs, which contain a GXXG canonical motif, that will be explained in further details in the next topic, instead this one has an <scene name='96/969643/Akea/2'>A-K-E-A</scene>. It is most likely involved in RNA binding and regulation, as seen in other proteins that also contain KH motifs. To give a more thorough response addressing the precise role played by the KH theme in FMRP, additional details would be needed. |
Overall, the FMRP NTD plays an important role as an RNA-binding protein, and its involvement in RNP complexes and its specific domains and motifs allow it to bind to specific RNAs and regulate their translation. <ref>MYRICK, L. K. et al. Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human Molecular Genetics, v. 24, n. 6, p. 1733–1740, 20 nov. 2014.[https://doi.org/10.1093/hmg/ddu586]</ref> | Overall, the FMRP NTD plays an important role as an RNA-binding protein, and its involvement in RNP complexes and its specific domains and motifs allow it to bind to specific RNAs and regulate their translation. <ref>MYRICK, L. K. et al. Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human Molecular Genetics, v. 24, n. 6, p. 1733–1740, 20 nov. 2014.[https://doi.org/10.1093/hmg/ddu586]</ref> | ||
| Line 43: | Line 43: | ||
==Central portion== | ==Central portion== | ||
| - | The central portion of the protein contains the ''in tandem'' KH1 AND KH2 | + | The central portion of the protein contains the ''in tandem'' <scene name='96/969643/Kh1_kh2/1'>KH1 AND KH2 domains</scene>. The type of KH domains in eukaryots is type 1 fold, present in eukaryotes, in which a <scene name='96/969643/Sheets_helices_kh1_kh2/1'>β sheet composed of three antiparallel strands is abutted by three helices</scene>, and they both have a canonical <scene name='96/969643/Gxxg/1'>C-X-X-G</scene> (glycine followed by any two aminoacids and another glycine) conserved domain, highlighted in magenta, connecting the central helices of the domain and a variable loop. There is a gap in the KH motif, which can hold '''four nucleic acid bases'''; it happens when an alpha-helix 1, alpha-helix 2, a GXXG on the left, and a variable loop are present in the domain, in the case of the FMRP another beta-sheet. This helps the FMRP in its RBP function, as they are motifs that are related to RNA or ssDNA binding. <ref> Valverde, R., Edwards, L. and Regan, L. (2008). Structure and function of KH domains. FEBS Journal, 275(11), pp.2712–2726. [https://doi.org/10.1111/j.1742-4658.2008.06411.x]</ref> |
---- | ---- | ||
Revision as of 00:21, 22 June 2023
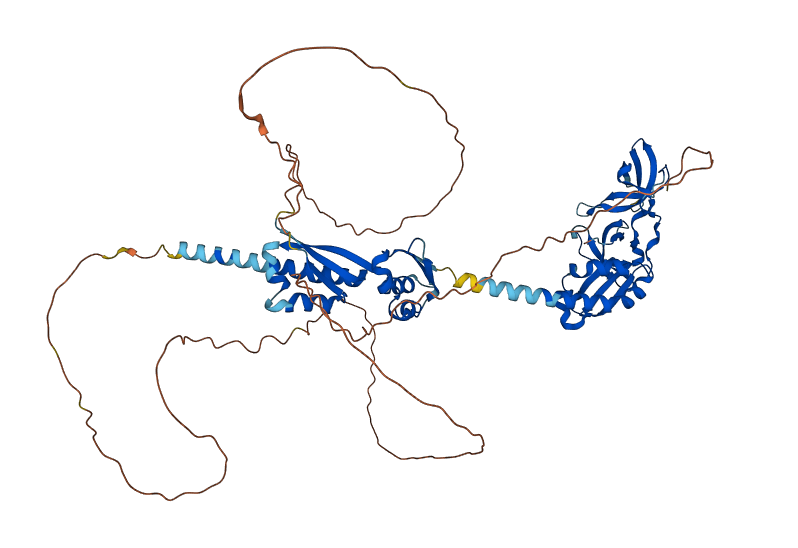
Structure of FMRP
Predicted FMRP
Fragile X messenger ribonucleoprotein (FMRP) is encoded by the fragile X messenger ribonucleoprotein 1 (FMR1) gene, located in the X chromosome, and is associated with the fragile X syndrome (FXS), Fragile X Tremor/Ataxia Syndrome (FXTAS) and Premature Ovarian Failure (POF1). FMRP functions as a synaptic regulator by binding to mRNAs and inhibiting its translation, therefore regulating the synthesis of proteins in the synapse. It is also an RNA binding protein, which is responsible for the transportation of mRNAs to the cytoplasm. The FMRP can also bind to its own FMR1 transcripts, possibly as a self-regulatory mechanism.
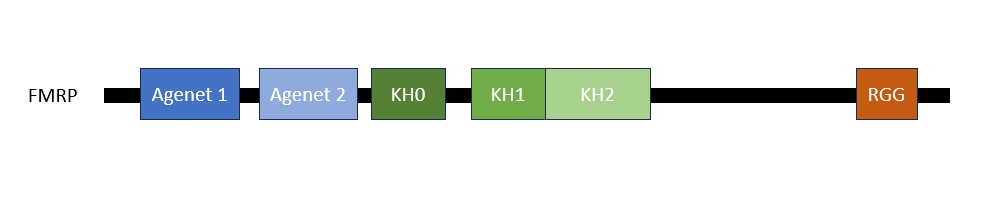
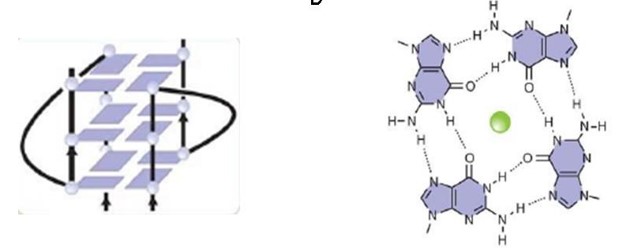
The FMRP is highly expressed in neurons and genitalia, and it's located mostly in the cytoplasm and lower levels in the nucleus. It contains domains related to its RNA binding function, either in the N-terminal or C-terminal domain; the Agenet and the KH0-motif are located in the N-terminal domain, and they, respectively, exerce functions in binding to methylated lysin and RNA binding; the KH1 and KH2 motifs are located in the central region of the protein; and the RGG box, in the C-terminal domain, acts as a binding to RNA, especifically to G-quadruplexes, a secondary RNA structure. The KH1, KH2 and RGG box domains allow the FMRP to bind and translate a number of mRNAs related to the synaptic plasticity. [1]
The protein has 20 non-redundant isoforms and the most common is isoform 7, and the longest isoform contains 632 aminoacids. [2].
The predicted image was generated from Ensembl, by the AlphaFold program.
Overall structure
| |||||||||||