We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Valentina Dutton/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
The vast majority of mitochondrial proteins have an N-terminal sequence that indicates that they must be exported to the mitochondria. This sequence is called the mitochondrial signal peptide. When the protein enters the mitochondria, this sequence is usually cleaved, providing greater stability. It is still unknown what this sequence would be in the case of PGAM5 since the protein signal peptide is not cleaved<ref name="sie">PMID:35921890</ref> and PGAM5 is anchored in its entirety to the inner membrane through its transmembrane domain, defined by amino acids 9-29<ref name="cha">PMID:28648608</ref>. | The vast majority of mitochondrial proteins have an N-terminal sequence that indicates that they must be exported to the mitochondria. This sequence is called the mitochondrial signal peptide. When the protein enters the mitochondria, this sequence is usually cleaved, providing greater stability. It is still unknown what this sequence would be in the case of PGAM5 since the protein signal peptide is not cleaved<ref name="sie">PMID:35921890</ref> and PGAM5 is anchored in its entirety to the inner membrane through its transmembrane domain, defined by amino acids 9-29<ref name="cha">PMID:28648608</ref>. | ||
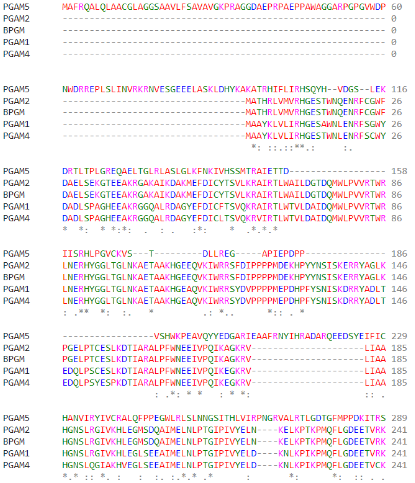
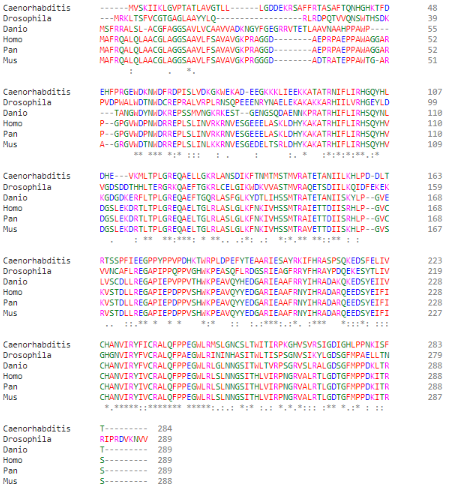
| - | Regarding the catalytic activity, <scene name='96/969636/His105/3'>histidine 5</scene> is responsible for the nucleophilic attack of the phosphate of the target protein, performing the intermediate link between the protein and the phosphate. However, histidine 105 is part of the canonical RHGE motif, present in | + | Regarding the catalytic activity, <scene name='96/969636/His105/3'>histidine 5</scene> is responsible for the nucleophilic attack of the phosphate of the target protein, performing the intermediate link between the protein and the phosphate. However, histidine 105 is part of the canonical RHGE motif, present in most proteins of the PGAM family, forming part of the PGAM domain (98-289)<ref name="cha" />. Protein <scene name='96/969636/Pgam5_dodecamer/2'>oligomerization</scene> is induced by the <scene name='96/969636/Wdxnwd/2'>WDXNWD motif</scene> at amino acids 58-63 which has been shown to function as an allosteric regulator of the specific phosphatase activity of PGAM5<ref name="wil" />. Mutations in this motif prevent oligomerization of the enzyme but still present as <scene name='96/969636/Pgam5_dimer/4'>dimers</scene> since the <scene name='96/969636/Dimerization_motif/2'>C-terminal tail</scene> (270-289) in PGAM5 is responsible for the dimerization of the protein<ref name="cha" />. These dimers, however, do not show phosphatase activity<ref name="wil" />. |
== Function == | == Function == | ||
Even though <scene name='96/969636/Pgam5_monomere/4'>PGAM5</scene> is a member of the PGAM protein family, it appears to lack phosphoglycerate mutase typical phosphotransferase and/or phosphohydrolase activities.<ref name="cha" />(UniProt). Instead, this protein is a serine/threonine (Ser/Thr) phosphatase, that is, it’s responsible for protein-protein interactions through dephosphorylation of serine/threonine and, occasionally, histidine residues<ref name="che">PMID:33370650</ref><ref name="shi">PMID:19879837</ref>. Its active site is composed of a histidine residue (His-105) responsible for the nucleophilic attack of the phosphorus atom acting as a phospho-acceptor<ref name="cha" /><ref name="shi" />. | Even though <scene name='96/969636/Pgam5_monomere/4'>PGAM5</scene> is a member of the PGAM protein family, it appears to lack phosphoglycerate mutase typical phosphotransferase and/or phosphohydrolase activities.<ref name="cha" />(UniProt). Instead, this protein is a serine/threonine (Ser/Thr) phosphatase, that is, it’s responsible for protein-protein interactions through dephosphorylation of serine/threonine and, occasionally, histidine residues<ref name="che">PMID:33370650</ref><ref name="shi">PMID:19879837</ref>. Its active site is composed of a histidine residue (His-105) responsible for the nucleophilic attack of the phosphorus atom acting as a phospho-acceptor<ref name="cha" /><ref name="shi" />. | ||
| Line 51: | Line 51: | ||
It’s suggested that PGAM5 might possibly be associated with diseases caused by mitochondrial dysfunction, especially if it occurs in response to defective mitophagy process, including neurodegenerative diseases<ref name="lia">PMID:34630036</ref><ref name="lue">PMID:25222142</ref>. | It’s suggested that PGAM5 might possibly be associated with diseases caused by mitochondrial dysfunction, especially if it occurs in response to defective mitophagy process, including neurodegenerative diseases<ref name="lia">PMID:34630036</ref><ref name="lue">PMID:25222142</ref>. | ||
| - | One such example is Parkinson’s disease, in which dopaminergic neurons die, likely due to oxidative stress and/or mitophagy defects. One of the proposed causes of Parkinson’s disease is a recessive mutation in autosomal gene responsible for the expression of PTENinduced putative kinase 1 (PINK1), a cytosolic and mitochondrial-associated kinase that regulates and promotes mitophagy in healthy cells, selectively eliminating dysfunctional mitochondria. This is mediated by accumulation of PINK1 in the mitochondrial outer membrane upon mitochondrial damage, which then recruits the E3 ubiquitin ligase Parkin, responsible for ubiquitinating MOM proteins and inducing degradation of mitochondria<ref name="lia" />. Furthermore, PGAM5 has also been reported as a regulator of PINK1/Parkin mediated mitophagy in mice, stabilizing said proteins, by binding to PINK1 and protecting it from proteases<ref name="lue" />. PGAM5 knockout (KO) mice have shown an almost total loss of mRNA nor protein expression, and these mice exhibited disrupted PINK1 stabilization and recruitment. Said mice were shown to have some, but not all, of the behavioral phenotype typical to Parkinson’s disease through dopaminergic neurodegeneration. It is therefore possible that deficiency in PGAM5 expression may be heavily associated with Parkinson’s disease and similar neurodegenerative conditions<ref name="lue" />. | + | One such example is Parkinson’s disease, in which dopaminergic neurons die, likely due to oxidative stress and/or mitophagy defects. One of the proposed causes of Parkinson’s disease is a recessive mutation in autosomal gene responsible for the expression of PTENinduced putative kinase 1 (PINK1), a cytosolic and mitochondrial-associated kinase that regulates and promotes mitophagy in healthy cells, selectively eliminating dysfunctional mitochondria. This is mediated by accumulation of PINK1 in the mitochondrial outer membrane upon mitochondrial damage, which then recruits the E3 ubiquitin ligase [[Parkin]], responsible for ubiquitinating MOM proteins and inducing degradation of mitochondria<ref name="lia" />. Furthermore, PGAM5 has also been reported as a regulator of PINK1/Parkin mediated mitophagy in mice, stabilizing said proteins, by binding to PINK1 and protecting it from proteases<ref name="lue" />. PGAM5 knockout (KO) mice have shown an almost total loss of mRNA nor protein expression, and these mice exhibited disrupted PINK1 stabilization and recruitment. Said mice were shown to have some, but not all, of the behavioral phenotype typical to Parkinson’s disease through dopaminergic neurodegeneration. It is therefore possible that deficiency in PGAM5 expression may be heavily associated with Parkinson’s disease and similar neurodegenerative conditions<ref name="lue" />. |
On the other hand, it is also possible that higher levels of PGAM5 may lead to diseases and complications. It has been studied that PGAM5 plays a role in non-small-cell lung cancer (NSCLC), albeit the mechanisms behind remain uncertain. It’s well documented that chronic obstructive pulmonary disease (COPD) patients have a higher risk of developing NSCLC, and that patients with NSCLC with established COPD have worse prognosis. Moreover, it is well recorded that oxidative stress contributes to the development of carcinogenic cells. In this sense, studies have reported that at least fourteen mitochondrial-related genes showed a fold difference in expression between non-cancerous and NSCLC cells, including PGAM5. This protein shows a large increase in not only mRNA expression but also protein levels in different types of lung cancer tissue compared to normal lung tissue. It’s still unknown how the overexpression of PGAM5 is related to lung cancer, though it is possible that the higher expression levels may lead to secondary uncontrolled cellular proliferation<ref name="ngk">PMID:30526542</ref>. | On the other hand, it is also possible that higher levels of PGAM5 may lead to diseases and complications. It has been studied that PGAM5 plays a role in non-small-cell lung cancer (NSCLC), albeit the mechanisms behind remain uncertain. It’s well documented that chronic obstructive pulmonary disease (COPD) patients have a higher risk of developing NSCLC, and that patients with NSCLC with established COPD have worse prognosis. Moreover, it is well recorded that oxidative stress contributes to the development of carcinogenic cells. In this sense, studies have reported that at least fourteen mitochondrial-related genes showed a fold difference in expression between non-cancerous and NSCLC cells, including PGAM5. This protein shows a large increase in not only mRNA expression but also protein levels in different types of lung cancer tissue compared to normal lung tissue. It’s still unknown how the overexpression of PGAM5 is related to lung cancer, though it is possible that the higher expression levels may lead to secondary uncontrolled cellular proliferation<ref name="ngk">PMID:30526542</ref>. | ||
| Line 59: | Line 59: | ||
Additionally, it’s possible for PGAM5 to be associated with female infertility. Ovarian aging presents changes in the quantity and quality of oocyte reserves over time, which can cause fertility issues, especially age related. Oocyte senescence is correlated with higher oxidative stress and possible mitochondrial dysfunction, leading to energy insufficiency necessary for fertilization to occur. When studying several infertility patients, a positive correlation between age of cumulus and granulosa cells and the expression levels of PGAM5 and its co-regulators. Furthermore, the quality of the mitochondria is decreased in aged mice, and PGAM5 knockdown reduces the aging process. Similarly to AIH and NSCLC, overexpression of PGAM5 seems to disturb oocyte mitochondrial activity, and although it’s still not elucidated how this protein acts in this scenario, PGAM5 has enormous potential to be a diagnostic marker for possible infertility patients<ref name="lie">PMID:34995407</ref>. | Additionally, it’s possible for PGAM5 to be associated with female infertility. Ovarian aging presents changes in the quantity and quality of oocyte reserves over time, which can cause fertility issues, especially age related. Oocyte senescence is correlated with higher oxidative stress and possible mitochondrial dysfunction, leading to energy insufficiency necessary for fertilization to occur. When studying several infertility patients, a positive correlation between age of cumulus and granulosa cells and the expression levels of PGAM5 and its co-regulators. Furthermore, the quality of the mitochondria is decreased in aged mice, and PGAM5 knockdown reduces the aging process. Similarly to AIH and NSCLC, overexpression of PGAM5 seems to disturb oocyte mitochondrial activity, and although it’s still not elucidated how this protein acts in this scenario, PGAM5 has enormous potential to be a diagnostic marker for possible infertility patients<ref name="lie">PMID:34995407</ref>. | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 02:17, 22 June 2023
Human Phosphoglycerate Mutase Family Member 5 (PGAM5)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Takeda K, Komuro Y, Hayakawa T, Oguchi H, Ishida Y, Murakami S, Noguchi T, Kinoshita H, Sekine Y, Iemura S, Natsume T, Ichijo H. Mitochondrial phosphoglycerate mutase 5 uses alternate catalytic activity as a protein serine/threonine phosphatase to activate ASK1. Proc Natl Acad Sci U S A. 2009 Jul 28;106(30):12301-5. Epub 2009 Jul 9. PMID:19590015 doi:http://dx.doi.org/0901823106
- ↑ 2.0 2.1 2.2 Wilkins JM, McConnell C, Tipton PA, Hannink M. A conserved motif mediates both multimer formation and allosteric activation of phosphoglycerate mutase 5. J Biol Chem. 2014 Sep 5;289(36):25137-48. PMID:25012655 doi:10.1074/jbc.M114.565549
- ↑ 3.0 3.1 Siebert V, Silber M, Heuten E, Muhle-Goll C, Lemberg MK. Cleavage of mitochondrial homeostasis regulator PGAM5 by the intramembrane protease PARL is governed by transmembrane helix dynamics and oligomeric state. J Biol Chem. 2022 Jul 31:102321. doi: 10.1016/j.jbc.2022.102321. PMID:35921890 doi:http://dx.doi.org/10.1016/j.jbc.2022.102321
- ↑ 4.0 4.1 4.2 4.3 4.4 Chaikuad A, Filippakopoulos P, Marcsisin SR, Picaud S, Schroder M, Sekine S, Ichijo H, Engen JR, Takeda K, Knapp S. Structures of PGAM5 Provide Insight into Active Site Plasticity and Multimeric Assembly. Structure. 2017 Jul 5;25(7):1089-1099.e3. doi: 10.1016/j.str.2017.05.020. Epub, 2017 Jun 22. PMID:28648608 doi:http://dx.doi.org/10.1016/j.str.2017.05.020
- ↑ Cheng M, Lin N, Dong D, Ma J, Su J, Sun L. PGAM5: A crucial role in mitochondrial dynamics and programmed cell death. Eur J Cell Biol. 2021 Jan;100(1):151144. PMID:33370650 doi:10.1016/j.ejcb.2020.151144
- ↑ 6.0 6.1 Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009 Oct 30;139(3):468-84. PMID:19879837 doi:10.1016/j.cell.2009.10.006
- ↑ 7.0 7.1 Lo SC, Hannink M. PGAM5, a Bcl-XL-interacting protein, is a novel substrate for the redox-regulated Keap1-dependent ubiquitin ligase complex. J Biol Chem. 2006 Dec 8;281(49):37893-903. PMID:17046835 doi:10.1074/jbc.M606539200
- ↑ Lo SC, Hannink M. PGAM5 tethers a ternary complex containing Keap1 and Nrf2 to mitochondria. Exp Cell Res. 2008 May 1;314(8):1789-803. doi: 10.1016/j.yexcr.2008.02.014. Epub , 2008 Mar 5. PMID:18387606 doi:http://dx.doi.org/10.1016/j.yexcr.2008.02.014
- ↑ 9.0 9.1 9.2 Baba T, Tanimura S, Yamaguchi A, Horikawa K, Yokozeki M, Hachiya S, Iemura SI, Natsume T, Matsuda N, Takeda K. Cleaved PGAM5 dephosphorylates nuclear serine/arginine-rich proteins during mitophagy. Biochim Biophys Acta Mol Cell Res. 2021 Jun;1868(7):119045. PMID:33872670 doi:10.1016/j.bbamcr.2021.119045
- ↑ 10.0 10.1 Liang MZ, Ke TL, Chen L. Mitochondrial Protein PGAM5 Emerges as a New Regulator in Neurological Diseases. Front Mol Neurosci. 2021 Sep 23;14:730604. PMID:34630036 doi:10.3389/fnmol.2021.730604
- ↑ 11.0 11.1 11.2 Lu W, Karuppagounder SS, Springer DA, Allen MD, Zheng L, Chao B, Zhang Y, Dawson VL, Dawson TM, Lenardo M. Genetic deficiency of the mitochondrial protein PGAM5 causes a Parkinson's-like movement disorder. Nat Commun. 2014 Sep 15;5:4930. PMID:25222142 doi:10.1038/ncomms5930
- ↑ Ng Kee Kwong F, Nicholson AG, Pavlidis S, Adcock IM, Chung KF. PGAM5 expression and macrophage signatures in non-small cell lung cancer associated with chronic obstructive pulmonary disease (COPD). BMC Cancer. 2018 Dec 10;18(1):1238. PMID:30526542 doi:10.1186/s12885-018-5140-9
- ↑ He GW, Günther C, Kremer AE, Thonn V, Amann K, Poremba C, Neurath MF, Wirtz S, Becker C. PGAM5-mediated programmed necrosis of hepatocytes drives acute liver injury. Gut. 2017 Apr;66(4):716-723. PMID:27566130 doi:10.1136/gutjnl-2015-311247
- ↑ Li CJ, Lin LT, Tsai HW, Wen ZH, Tsui KH. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell. 2022 Feb;21(2):e13546. PMID:34995407 doi:10.1111/acel.13546