We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Marcella Maringolo/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | It was hypothesized that NF-YA is recruited by the HFD domain because it would function as a histone tail to allow and stabilize DNA binding with the NF-Y complex. Indeed, the core structures the histone dimer H2A/H2B overlay with NF-YB and NF-YC, while NF-YA overlay with the HB2 tail, indicating a similar function for those structures. | ||
Therefore, the main function of NF-Y is related to its high specificity and affinity binding to DNA, which induces gene promoter activation with the help of other transcriptional factors, although its binding can also induce posttranslational alterations in histones, such as methylations and acetylations, being also capable of establishing acetylation marks directly on gene promoters through recruitment of enzymes responsible for inducing those alterations. This epigenetic marks can induce or repress gene transcription by modifying chromatin accessibility. Hence, NF-Y can primary bind into a promoter region where transcription preinitiation complexes can be established, avoiding nucleosome formation and giving space for other transcription initiation proteins (some of those can interact directly with NF-Y and hence be recruited by it) and TFs to bind to the promoter of a gene that will be transcripted. It is hypothesized that, since NF-Y have a similar structure to histones, its binding to the DNA results in incompatibility for other histones binding to the same region. This primary binding also can recruit histone modifying enzymes, which regulate chromatin accessibility. Another possible function for NF-Y is that it can ensure the usage of the correct TSS site for transcription. It was shown that NF-YA depletion in a cell can cause histone binding to the correct TSS, which result in the relocation of the preinitiation complex to another TSS that is upstream of the canonical one. This shift in the position of the TSS can result in a longer mRNA that could be originated from another ORF, resulting in abnormal mRNAs. This function of NF-Y could be explained by the fact that this TF predominantly binds to CG-rich promoters of essential genes (such as those related to DNA repair, cell cycle and transcription regulation), which need to be transcripted correctly. | Therefore, the main function of NF-Y is related to its high specificity and affinity binding to DNA, which induces gene promoter activation with the help of other transcriptional factors, although its binding can also induce posttranslational alterations in histones, such as methylations and acetylations, being also capable of establishing acetylation marks directly on gene promoters through recruitment of enzymes responsible for inducing those alterations. This epigenetic marks can induce or repress gene transcription by modifying chromatin accessibility. Hence, NF-Y can primary bind into a promoter region where transcription preinitiation complexes can be established, avoiding nucleosome formation and giving space for other transcription initiation proteins (some of those can interact directly with NF-Y and hence be recruited by it) and TFs to bind to the promoter of a gene that will be transcripted. It is hypothesized that, since NF-Y have a similar structure to histones, its binding to the DNA results in incompatibility for other histones binding to the same region. This primary binding also can recruit histone modifying enzymes, which regulate chromatin accessibility. Another possible function for NF-Y is that it can ensure the usage of the correct TSS site for transcription. It was shown that NF-YA depletion in a cell can cause histone binding to the correct TSS, which result in the relocation of the preinitiation complex to another TSS that is upstream of the canonical one. This shift in the position of the TSS can result in a longer mRNA that could be originated from another ORF, resulting in abnormal mRNAs. This function of NF-Y could be explained by the fact that this TF predominantly binds to CG-rich promoters of essential genes (such as those related to DNA repair, cell cycle and transcription regulation), which need to be transcripted correctly. | ||
| + | |||
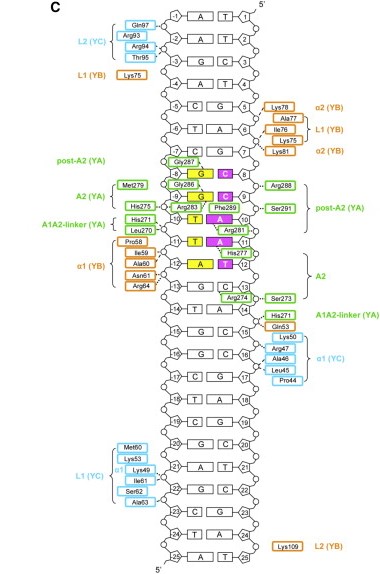
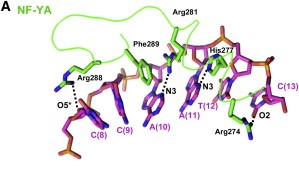
Here we show the <scene name='97/973093/Human_nf-y/2'>Human NF-Y</scene> molecule structure while binding to the DNA, with each subunit represented in a different color: NF-YA in blue, NF-YB in green and NF-YC in pink, while the DNA is represented in grey. Here is its <scene name='97/973093/Human_nf-y/9'>Backbone</scene> and its <scene name='97/973093/Secondary_structure/1'>Secondary structure</scene> with alpha-helixes colored in pink, and beta-sheets colored in yellow. Using the <scene name='97/973093/Cartoon/1'>cartoon</scene> representation we can see the secondary structures in each subunit. | Here we show the <scene name='97/973093/Human_nf-y/2'>Human NF-Y</scene> molecule structure while binding to the DNA, with each subunit represented in a different color: NF-YA in blue, NF-YB in green and NF-YC in pink, while the DNA is represented in grey. Here is its <scene name='97/973093/Human_nf-y/9'>Backbone</scene> and its <scene name='97/973093/Secondary_structure/1'>Secondary structure</scene> with alpha-helixes colored in pink, and beta-sheets colored in yellow. Using the <scene name='97/973093/Cartoon/1'>cartoon</scene> representation we can see the secondary structures in each subunit. | ||
| Line 18: | Line 19: | ||
== References == | == References == | ||
Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7. PMID: 31296853; PMCID: PMC6624317. | Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7. PMID: 31296853; PMCID: PMC6624317. | ||
| + | |||
Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047. | Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047. | ||
<references/> | <references/> | ||
Revision as of 13:39, 23 June 2023
| |||||||||||
References
Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7. PMID: 31296853; PMCID: PMC6624317.
Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047.