We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Marcella Maringolo/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
== Function and Structural highlights == | == Function and Structural highlights == | ||
| - | NF-Y is a highly conserved and ubiquitously expressed protein that acts as a transcription factor, binding directly to a specific site on the DNA, the CCAAT box, a sequence that can be found in the promoter of many protein coding genes. Studies show that the CCAAT sites in the genome are located upstream of the TSS site in the promoter of those genes. This protein is composed of 3 subunits, NF-YA (subunit Alpha), NF-YB (subunit Beta) and NF-YC (subunit Gamma), which are all necessary for DNA binding. The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. NF-YB and NF-YC show homology in sequence with histones H2A and H2B, respectively, hence, the Beta and Gamma subunits can bind directly to the DNA, but require NF-YA association to stabilize the binding of the complex to the DNA. | + | The structure of the NF-Y protein described here was generated by a crystallography using synchrotron radiation at 3.1Å resolution. The molecule consists of a heterotrimer complexed with a 25bp DNA fragment containing a CCAAT sequence. |
| + | NF-Y is a highly conserved (strongly conserved in all eukaryotes) and ubiquitously expressed protein that acts as a transcription factor, binding directly to a specific site on the DNA, the CCAAT box, a sequence that can be found in the promoter of many protein coding genes. Studies show that the CCAAT sites in the genome are located upstream of the TSS site in the promoter of those genes. This protein is composed of 3 subunits, NF-YA (subunit Alpha), NF-YB (subunit Beta) and NF-YC (subunit Gamma), which are all necessary for DNA binding. The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. NF-YB and NF-YC show homology in sequence with histones H2A and H2B, respectively, hence, the Beta and Gamma subunits can bind directly to the DNA, but require NF-YA association to stabilize the binding of the complex to the DNA. | ||
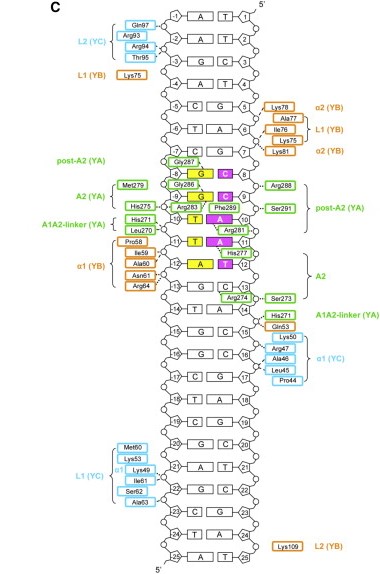
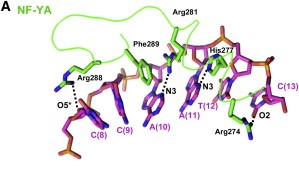
The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | ||
| Line 15: | Line 16: | ||
Here we show the <scene name='97/973093/Human_nf-y/2'>Human NF-Y</scene> molecule structure while binding to the DNA, with each subunit represented in a different color: NF-YA in blue, NF-YB in green and NF-YC in pink, while the DNA is represented in grey. Here is its <scene name='97/973093/Human_nf-y/9'>Backbone</scene> and its <scene name='97/973093/Secondary_structure/1'>Secondary structure</scene> with alpha-helixes colored in pink, and beta-sheets colored in yellow. Using the <scene name='97/973093/Cartoon/1'>cartoon</scene> representation we can see the secondary structures in each subunit. | Here we show the <scene name='97/973093/Human_nf-y/2'>Human NF-Y</scene> molecule structure while binding to the DNA, with each subunit represented in a different color: NF-YA in blue, NF-YB in green and NF-YC in pink, while the DNA is represented in grey. Here is its <scene name='97/973093/Human_nf-y/9'>Backbone</scene> and its <scene name='97/973093/Secondary_structure/1'>Secondary structure</scene> with alpha-helixes colored in pink, and beta-sheets colored in yellow. Using the <scene name='97/973093/Cartoon/1'>cartoon</scene> representation we can see the secondary structures in each subunit. | ||
| + | |||
| + | ==NF-Y and diseases== | ||
| + | |||
| + | It was shown that NF-Y binding sites are abundant in promoters of genes that induce cell growth or that are responsible for cell transformation. Therefore, NF-Y could induce transcription of oncogenes, leading to cell growth and transformation in normal cells, which can result in tumor-like cells. Overexpression of NF-Y subunits was already described in different types of cancers such as lung, breast, thyroid and prostate cancer. | ||
| + | NF-Y subunits like NF-YA and NF-YC can form aggregates with mutant huntingtin (HTT) protein, which is abundant in neurons of patients with Hungtinton’s disease. By sequestering NF-Y subunits, mutant HTT impairs NF-Y dependent transcriptional activation of genes, such as HSP70, that encodes for a chaperone protein responsible for degradation of mutant HTT aggregates. In conclusion, NF-Y is required for adequate transcription of chaperones, and its absence in the CCAAT sites can induce aggregates accumulation, which aggravates the disease. | ||
| + | This molecule can also be involved in diabetes, when its DNA binding in promoter of enzymes related to glucose metabolism is either reduced or augmented, causing non ideal transcription of the downstream genes, which impact in regulation of blood glucose levels. | ||
| + | Other circumstances where mutations of the NF-Y binding site occur can result in the block of NF-Y binding, altering the expression of genes regulated by NF-Y transcriptional regulation. | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Revision as of 20:12, 24 June 2023
| |||||||||||
References
Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7. PMID: 31296853; PMCID: PMC6624317.
Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047.