User:Marcella Maringolo/Sandbox 1
From Proteopedia
| Line 7: | Line 7: | ||
[[Image:CHARTNFY.png]] | [[Image:CHARTNFY.png]] | ||
| + | NF-Y, largely described as a transcription activator via its promoter-proximal binding, is a key regulator of cell cycle progression in proliferating cells, with its activity often downregulated during cellular differentiation and senescence. In addition to binding core promoters, NF-Y has also been shown to bind enhancer elements away from TSSs, but its function and mechanism of action at these distal regulatory elements remain to be elucidated. | ||
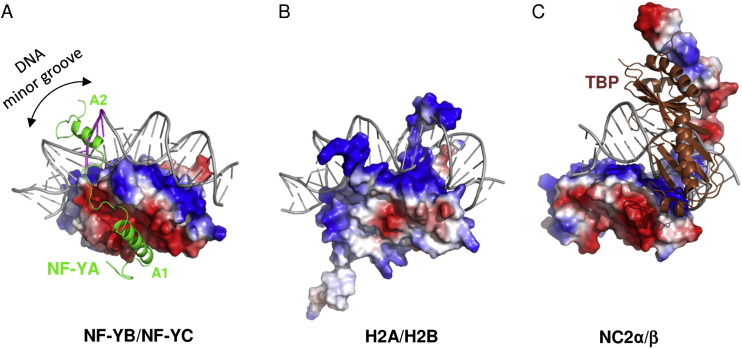
| - | The key structural feature of the NF-Y/DNA complex is the minor-groove interaction of NF-YA, which induces an approximately 80° bend in the DNA. The structure and DNA-binding mode of NF-YB/NF-YC H FDs are similar to those of the core histones H2A/H2B, TATA-binding protein (TBP)-associated factors (TAFs), the TBP/ TATA-binding negative cofactor 2 (NC2α/β), and the CHRAC15/CHRAC17 subunits of the nucleosome remodeling complex CHRAC. Yet, unlike H2A/H2B which lack sequence specificity, NF-YB/NF-YC interaction with NF-YA provides the NF-Y complex with sequence-specific targeting capability as well as nucleosome-like properties of non-specific DNA binding, a combination that allows for stable DNA binding. NF-Y, largely described as a transcription activator via its promoter-proximal binding, is a key regulator of cell cycle progression in proliferating cells, with its activity often downregulated during cellular differentiation and senescence. In addition to binding core promoters, NF-Y has also been shown to bind enhancer elements away from TSSs, but its function and mechanism of action at these distal regulatory elements remain to be elucidated. The image below shows the difference between factors cited on text. | ||
| - | |||
| - | [[Image: Diferences.jpg]] | ||
== Function and Structural highlights == | == Function and Structural highlights == | ||
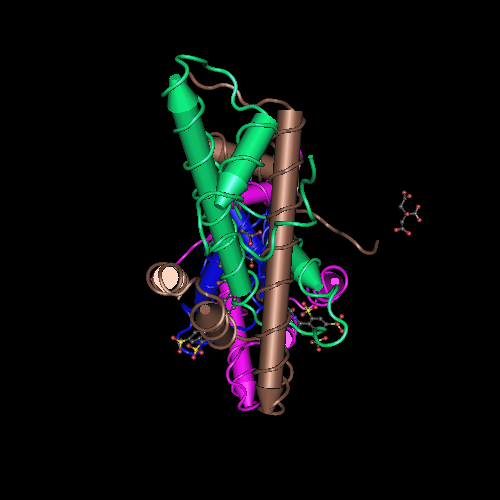
The structure of the NF-Y protein described here was generated by a crystallography using synchrotron radiation at 3.1Å resolution. The molecule consists of a heterotrimer complexed with a 25bp DNA fragment containing a CCAAT sequence. | The structure of the NF-Y protein described here was generated by a crystallography using synchrotron radiation at 3.1Å resolution. The molecule consists of a heterotrimer complexed with a 25bp DNA fragment containing a CCAAT sequence. | ||
| - | NF-Y is a highly conserved (strongly conserved in all eukaryotes) and ubiquitously expressed protein that acts as a transcription factor, binding directly to a specific site on the DNA, the CCAAT box, a sequence that can be found in the promoter of many protein coding genes. Studies show that the CCAAT sites in the genome are located upstream of the TSS site in the promoter of those genes. This protein is composed of 3 subunits, NF-YA (subunit Alpha), NF-YB (subunit Beta) and NF-YC (subunit Gamma), which are all necessary for DNA binding. The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. NF-YB | + | NF-Y is a highly conserved (strongly conserved in all eukaryotes) and ubiquitously expressed protein that acts as a transcription factor, binding directly to a specific site on the DNA, the CCAAT box, a sequence that can be found in the promoter of many protein coding genes. Studies show that the CCAAT sites in the genome are located upstream of the TSS site in the promoter of those genes. This protein is composed of 3 subunits, NF-YA (subunit Alpha), NF-YB (subunit Beta) and NF-YC (subunit Gamma), which are all necessary for DNA binding. The NF-YB and NF-YC subunits host histone fold-domains (HFDs), which are present in the core of histones and allow binding to the DNA. The structure and DNA-binding mode of NF-YB/NF-YC are similar to those of the core histones H2A/H2B, TATA-binding protein (TBP)-associated factors (TAFs), the TBP/ TATA-binding negative cofactor 2 (NC2α/β), and the CHRAC15/CHRAC17 subunits of the nucleosome remodeling complex CHRAC. But the biggest difference between them is the need for the presence of NF-YA association to stabilize the binding of the complex to the DNA. The image below shows the difference between factors cited on text. |
| + | |||
| + | [[Image: Diferences.jpg]] | ||
| + | |||
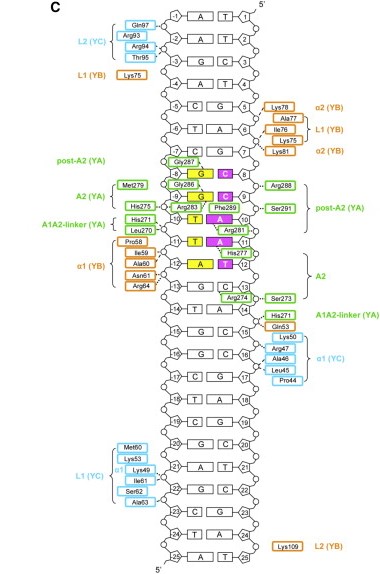
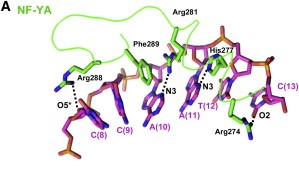
The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | The heterodimerization of NF-YB and NF-YC allow NF-YA to associate with the complex, through binding of its A1 helix to NF-YB and NF-YC HFD domains, then allowing binding to CCAAT. NF-YA has a specific C-terminal domain responsible for CCAAT recognition, its A2 helix, which searches for the DNA motif for binding, but all of the 3 subunits can contact DNA after CCAAT binding. The contact regions for NF-Y subunits are shown in the image below. | ||
Revision as of 14:41, 25 June 2023
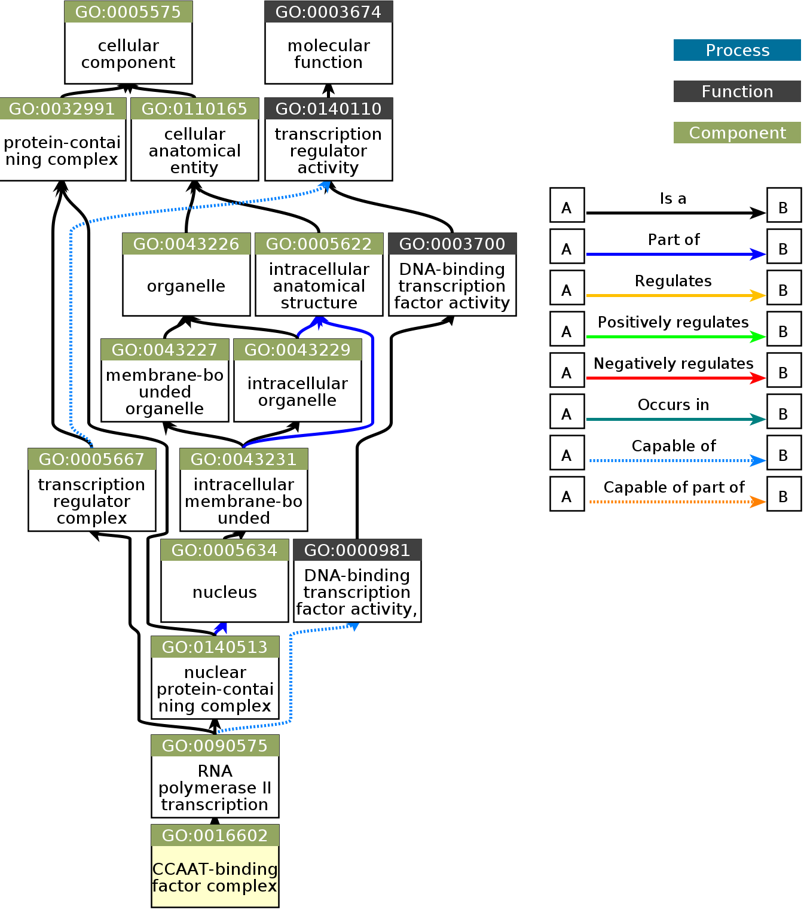
| |||||||||||
References
Dolfini D, Gatta R, Mantovani R. NF-Y and the transcriptional activation of CCAAT promoters. Crit Rev Biochem Mol Biol. 2012 Jan-Feb;47(1):29-49. doi: 10.3109/10409238.2011.628970.
McNabb DS, Xing Y, Guarente L. Cloning of yeast HAP5: a novel subunit of a heterotrimeric complex required for CCAAT binding. Genes & Development. 1995 Jan;9(1):47-58. DOI: 10.1101/gad.9.1.47. PMID: 7828851.
Nardini, Marco, et al. “Sequence-Specific Transcription Factor NF-Y Displays Histone-like DNA Binding and H2B-like Ubiquitination.” Cell, vol. 152, no. 1-2, 17 Jan. 2013, pp. 132–143, https://doi.org/10.1016/j.cell.2012.11.047.
Oldfield AJ, Henriques T, Kumar D, Burkholder AB, Cinghu S, Paulet D, Bennett BD, Yang P, Scruggs BS, Lavender CA, Rivals E, Adelman K, Jothi R. NF-Y controls fidelity of transcription initiation at gene promoters through maintenance of the nucleosome-depleted region. Nat Commun. 2019 Jul 11;10(1):3072. doi: 10.1038/s41467-019-10905-7.
Structural Basis of Inhibition of the Pioneer Transcription Factor NF-Y by Suramin Nardone V, Chaves-Sanjuan A, Lapi M, Airoldi C, Saponaro A, Pasqualato S, Dolfini D, Camilloni C, Bernardini A, Gnesutta N, Mantovani R, Nardini M