User:Marcos Vinícius Caetano/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Function == | == Function == | ||
| - | Myosins (including Myosin VI) are involved in a wide variety of functions, such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins. | + | Myosins (including Myosin VI) are involved in a '''wide variety of functions''', such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins. |
== Structure == | == Structure == | ||
| Line 19: | Line 19: | ||
| + | Predicted amino acid sequences and cDNA comparison of Myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region. This is probably due to the unique and different functions of this protein. This scene represents the <scene name='97/973101/Conservation/1'>Myosin VI evolutionary conservation</scene>, where <span style="color:pink">'''pink gradient'''</span> shows the more conserved region, <span style="color:grey">'''white'''</span> is average, <span style="color:blue">'''blue gradient'''</span> is the variable region and <span style="color:grey">'''grey'''</span> or <span style="color:yellow">'''yellow'''</span> is insufficient data. | ||
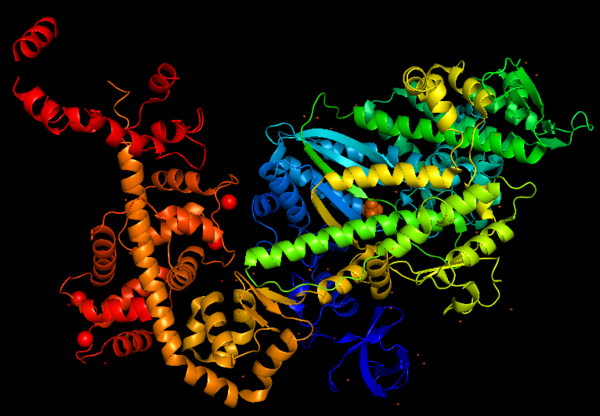
This figure portrays Myosin VI structure in terms of alpha helix (in <span style="color:red">'''red'''</span>) and β-sheet (in <span style="color:yellow">'''yellow'''</span>). The distortion of the central β-sheet differs depending on the states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi), and from other classes of myosin. | This figure portrays Myosin VI structure in terms of alpha helix (in <span style="color:red">'''red'''</span>) and β-sheet (in <span style="color:yellow">'''yellow'''</span>). The distortion of the central β-sheet differs depending on the states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi), and from other classes of myosin. | ||
| Line 24: | Line 25: | ||
[[Image:MyoVI alpha and beta v2.png|600px]] | [[Image:MyoVI alpha and beta v2.png|600px]] | ||
| - | |||
| - | Predicted amino acid sequences and cDNA comparison of Myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region. This is probably due to the unique and different functions of this protein. This scene represents the <scene name='97/973101/Conservation/1'>Myosin VI evolutionary conservation</scene>, where <span style="color:pink">'''pink gradient'''</span> shows the more conserved region, <span style="color:grey">'''white'''</span> is average, <span style="color:blue">'''blue gradient'''</span> is the variable region and <span style="color:grey">'''grey'''</span> or <span style="color:yellow">'''yellow'''</span> is insufficient data. | ||
Revision as of 22:51, 25 June 2023
Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure
| |||||||||||