User:Marcos Vinícius Caetano/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
[[Image:MyoVI.png|600px]] | [[Image:MyoVI.png|600px]] | ||
| - | == Function == | ||
| - | Myosins (including Myosin VI) are involved in a '''wide variety of functions''', such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins. | ||
| - | |||
| - | == Structure == | ||
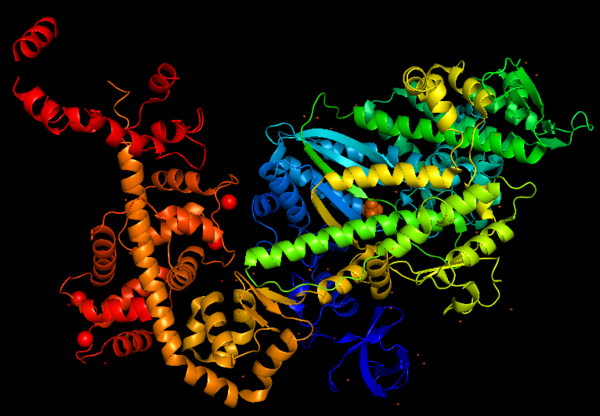
The basic structure of myosin VI, and most myosin molecule heavy chains, consists of '''three regions''': | The basic structure of myosin VI, and most myosin molecule heavy chains, consists of '''three regions''': | ||
*'''The catalytic motor head domain''' (NH2-terminal motor): responsible for binding to actin and hydrolysis of ATP. It is a highly conserved structure; | *'''The catalytic motor head domain''' (NH2-terminal motor): responsible for binding to actin and hydrolysis of ATP. It is a highly conserved structure; | ||
*'''The neck region''': contains the IQ domain/motif that bind light chains (calcium sensor calmodulin); | *'''The neck region''': contains the IQ domain/motif that bind light chains (calcium sensor calmodulin); | ||
*'''The tail domain''' (COOH-terminal): binds cargoes and anchors the protein to specific membrane compartments. | *'''The tail domain''' (COOH-terminal): binds cargoes and anchors the protein to specific membrane compartments. | ||
| - | |||
Predicted amino acid sequences and cDNA comparison of Myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region. This is probably due to the unique and different functions of this protein. This scene represents the <scene name='97/973101/Conservation/1'>Myosin VI evolutionary conservation</scene>, where <span style="color:pink">'''pink gradient'''</span> shows the more conserved region, <span style="color:grey">'''white'''</span> is average, <span style="color:blue">'''blue gradient'''</span> is the variable region and <span style="color:grey">'''grey'''</span> or <span style="color:yellow">'''yellow'''</span> is insufficient data. | Predicted amino acid sequences and cDNA comparison of Myosin VI in different species shows strong evolutionary conservation, mainly in the head/motor domain and in the distal tail region. This is probably due to the unique and different functions of this protein. This scene represents the <scene name='97/973101/Conservation/1'>Myosin VI evolutionary conservation</scene>, where <span style="color:pink">'''pink gradient'''</span> shows the more conserved region, <span style="color:grey">'''white'''</span> is average, <span style="color:blue">'''blue gradient'''</span> is the variable region and <span style="color:grey">'''grey'''</span> or <span style="color:yellow">'''yellow'''</span> is insufficient data. | ||
| + | |||
| + | == Function == | ||
| + | Myosins (including Myosin VI) are involved in a '''wide variety of functions''', such as cell migration and adhesion, cytokinesis, phagocytosis, maintenance of cell shape, signal transduction and intracellular transport and localization of organelles and macromolecules. Due to these diverse roles, each year more studies emerge with the objective to understand more of the structure, mechanisms and functions of myosins. | ||
| + | |||
| + | == Structure highlights == | ||
| + | The crystal structure of the entry '2BKI' was determined by x-ray diffraction with the resolution of 2,90 Å and is composed by 3 chains: <scene name='97/973101/Myo_vi_chain_a/1'>Myosin VI</scene> and two chains of <scene name='97/973101/Chains_b_d/1'>Calmodulin</scene>. | ||
This figure portrays Myosin VI structure in terms of alpha helix (in <span style="color:red">'''red'''</span>) and β-sheet (in <span style="color:yellow">'''yellow'''</span>). The distortion of the central β-sheet differs depending on the states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi), and from other classes of myosin. | This figure portrays Myosin VI structure in terms of alpha helix (in <span style="color:red">'''red'''</span>) and β-sheet (in <span style="color:yellow">'''yellow'''</span>). The distortion of the central β-sheet differs depending on the states of the motor, such as post-rigor (ATP) or pre-powerstroke (ADP.Pi), and from other classes of myosin. | ||
[[Image:MyoVI alpha and beta v2.png|600px]] | [[Image:MyoVI alpha and beta v2.png|600px]] | ||
| - | |||
| - | |||
| - | |||
| - | == Structure highlights == | ||
| - | The crystal structure of the entry '2BKI' was determined by x-ray diffraction with the resolution of 2,90 Å and is composed by 3 chains: <scene name='97/973101/Myo_vi_chain_a/1'>Myosin VI</scene> and two chains of <scene name='97/973101/Chains_b_d/1'>Calmodulin</scene>. | ||
The motor domain contains two inserts that are unique in the myosin superfamily: | The motor domain contains two inserts that are unique in the myosin superfamily: | ||
Revision as of 22:53, 25 June 2023
Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure
| |||||||||||