User:Davi de Souza/Sandbox 1
From Proteopedia
| Line 3: | Line 3: | ||
== General Aspects and function == | == General Aspects and function == | ||
| - | Aerolysin is a [[toxin]] synthesized by some species of bacteria belonging to the genus Aeromonas, such as Aeromonas hydrophila. The exact function of Aerolysin may vary among different species and strains of Aeromonas. However, it is evident that it is the main macromolecule responsible for the pathogenicity of Aeromonas hydrophila, being associated with diarrheal diseases and deep wound infections <ref>PMID: 2649316</ref>. About the structure of the protein, it is known that the toxin is setted up in an oligomeric structure with the activated forms of the monomers called proaerolysin. | + | Aerolysin is a [[toxin]] synthesized by some species of bacteria belonging to the genus ''Aeromonas'', such as ''Aeromonas hydrophila''. The exact function of Aerolysin may vary among different species and strains of ''Aeromonas''. However, it is evident that it is the main macromolecule responsible for the pathogenicity of ''Aeromonas hydrophila'', being associated with diarrheal diseases and deep wound infections <ref>PMID: 2649316</ref>. About the structure of the protein, it is known that the toxin is setted up in an oligomeric structure with the activated forms of the monomers called proaerolysin. |
| - | Aerolysin plays several roles in the pathogenicity of Aeromonas | + | Aerolysin plays several roles in the pathogenicity of ''Aeromonas sp''. One of its main functions is its ability to promote ''lysis'' (rupture) of host cells, such as epithelial cells and immune cells. Aerolysin exhibits cytotoxic activity, causing damage to the cell membranes of host cells, which can lead to cell death and contribute to the bacterium's pathogenicity. |
| - | Furthermore, aerolysin may be involved in the invasion and dissemination of the bacterium within the host. It can assist in tissue degradation, facilitating the bacterium's invasion into different organs and tissues of the host | + | Furthermore, aerolysin may be involved in the invasion and dissemination of the bacterium within the host. It can assist in tissue degradation, facilitating the bacterium's invasion into different organs and tissues of the host. Additionally, there are other proteins and virulence factors produced by ''Aeromonas spp''. that also play important roles in the pathogenicity of these bacteria. |
| - | + | ||
| Line 14: | Line 13: | ||
'''Functional domains and their roles''' | '''Functional domains and their roles''' | ||
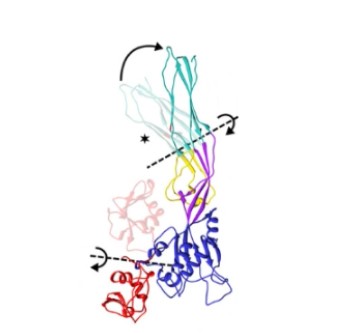
| - | Using the <scene name='97/973994/Proaerolysin/5'>monomer structure (proaerolysin)</scene> as a model of study, it is possible to see that the protein has <scene name='97/973994/Proaerolysin/6'>4 domains</scene>. Domains 1 and 2 are responsible for binding to N-glycosylated phosphatidylinositol (GPI) proteins, which are present in the membranes of eukaryotic cells. <scene name='97/973994/Proaerolysin_domain1/1'>Domain 1</scene> binds to specific sugar modifications found on the GPI, while <scene name='97/973994/Proaerolysin_domain_2/1'>domain 2</scene> directly binds to the glycan core located in the central portion of GPI proteins. Therefore, both domain 1 and domain 2 are involved in anchoring the aerolysin proprotein to the target cell membrane. <scene name='97/973994/Proaerolysin_domain_3/1'>Domain 3</scene> is composed of a five-stranded beta sheet and a <scene name='97/973994/Prestem_loop_domain_3/1'>pre-stem loop</scene>, which are important for the insertion and anchoring of the protein into the cell membrane. <scene name='97/973994/Proaerolysin_domain4/1'>Domain 4</scene> is an extension of the beta sheet from domain 3, but it is opened by the <scene name='97/973994/Proaerolysin_ctp/1'>C-terminal peptide (CTP)</scene>, forming a twisted double-fold in the beta sheet. This conformational change induced by the CTP propeptide allows the protein to fold into its soluble form.<ref>PMID: 7510043</ref> | + | Using the <scene name='97/973994/Proaerolysin/5'>monomer structure (''proaerolysin'')</scene> as a model of study, it is possible to see that the protein has <scene name='97/973994/Proaerolysin/6'>4 domains</scene>. Domains 1 and 2 are responsible for binding to N-glycosylated phosphatidylinositol (GPI) proteins, which are present in the membranes of eukaryotic cells. <scene name='97/973994/Proaerolysin_domain1/1'>Domain 1</scene> binds to specific sugar modifications found on the GPI, while <scene name='97/973994/Proaerolysin_domain_2/1'>domain 2</scene> directly binds to the glycan core located in the central portion of GPI proteins. Therefore, both domain 1 and domain 2 are involved in anchoring the aerolysin proprotein to the target cell membrane. <scene name='97/973994/Proaerolysin_domain_3/1'>Domain 3</scene> is composed of a five-stranded beta sheet and a <scene name='97/973994/Prestem_loop_domain_3/1'>pre-stem loop</scene>, which are important for the insertion and anchoring of the protein into the cell membrane. <scene name='97/973994/Proaerolysin_domain4/1'>Domain 4</scene> is an extension of the beta sheet from domain 3, but it is opened by the <scene name='97/973994/Proaerolysin_ctp/1'>C-terminal peptide (CTP)</scene>, forming a twisted double-fold in the beta sheet. This conformational change induced by the CTP propeptide allows the protein to fold into its soluble form.<ref>PMID: 7510043</ref> |
'''Structure highlights of the Aerolysin Oligomer''' | '''Structure highlights of the Aerolysin Oligomer''' | ||
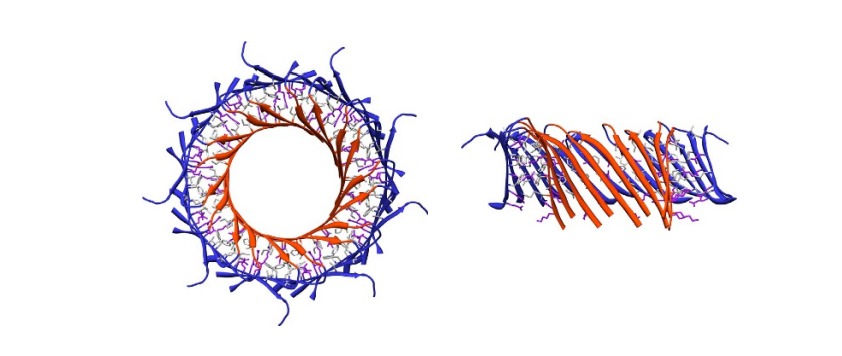
| - | When the CTP is cleaved from the main body of the protein, the | + | When the CTP is cleaved from the main body of the protein, the proaerolysin oligomerizes into a heptameric ring on the target cell membrane, forming a pore. During proaerolysin oligomerization, domain 1 undergoes rotation, allowing all receptor binding sites to position themselves correctly relative to the target membrane. Domains 2 and 3 remain largely unchanged. On the other hand, domain 4, after cleavage of the CTP propeptide, undergoes rearrangement of the beta sheets, forming a beta sandwich. In the core of this sandwich, hydrophobic residues are internalized. Additionally, domain 4 rotates relative to domain 3, enabling hydrogen bonding between the beta sandwiches of two monomers, promoting the oligomerization of aerolysin.<ref>PMID: 27405240</ref> |
[[Image:Torção 2.jpg]] | [[Image:Torção 2.jpg]] | ||
| Line 26: | Line 25: | ||
(Iacovache, I., De Carlo, S., Cirauqui, N. et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat Commun 7, 12062 (2016). https://doi.org/10.1038/ncomms12062) | (Iacovache, I., De Carlo, S., Cirauqui, N. et al. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat Commun 7, 12062 (2016). https://doi.org/10.1038/ncomms12062) | ||
| - | Subsequently, domains 3 and 4 twist slightly relative to domains 1 and 2, allowing for the elongation of the inner beta barrel through the addition of amino acids. In the 3D representation of the box next to it, we can observe the phenomenon described above: First showing the <scene name='97/973994/ | + | Subsequently, domains 3 and 4 twist slightly relative to domains 1 and 2, allowing for the elongation of the inner beta barrel through the addition of amino acids. In the 3D representation of the box next to it, we can observe the phenomenon described above: First showing the <scene name='97/973994/Beta_sheets_prepore/2'>beta sheets</scene> that form the cylindrical tube of the barrel and then the <scene name='97/973994/Domains_prepore/2'>post-pre-pore structure</scene>, with the domains of a monomer. |
Finally, the inner beta barrel elongates with the addition of an additional 15 residues in the pre-stem loop. Additionally, a twist occurs in two regions adjacent to domain 3, located at the base of the beta barrel. | Finally, the inner beta barrel elongates with the addition of an additional 15 residues in the pre-stem loop. Additionally, a twist occurs in two regions adjacent to domain 3, located at the base of the beta barrel. | ||
| Line 33: | Line 32: | ||
(Iacovache, I., De Carlo, S., Cirauqui, N. et al. A estrutura Cryo-EM de variantes de aerolisina revela uma nova dobra de proteína e o processo de formação de poros. Nat Commun 7, 12062 (2016). https: / /doi.org/10.1038/ncomms12062 ) | (Iacovache, I., De Carlo, S., Cirauqui, N. et al. A estrutura Cryo-EM de variantes de aerolisina revela uma nova dobra de proteína e o processo de formação de poros. Nat Commun 7, 12062 (2016). https: / /doi.org/10.1038/ncomms12062 ) | ||
| - | This twist results in a vertical collapse in the protein, resulting in the extension of the beta barrel. In the 3D representation of the box next to it, we can observe the phenomenon described above: first showing the <scene name='97/973994/ | + | This twist results in a vertical collapse in the protein, resulting in the extension of the beta barrel. In the 3D representation of the box next to it, we can observe the phenomenon described above: first showing the <scene name='97/973994/Beta_sheets_quasipore/2'>beta sheets</scene> that form the cylindrical tube of the barrel and then the <scene name='97/973994/Domains_quasipore/3'>quasipore structure</scene>, with the domains of a monomer. |
| - | + | ||
__NOTOC__ | __NOTOC__ | ||
== 3D Structures of Aerolysin == | == 3D Structures of Aerolysin == | ||
Revision as of 02:43, 26 June 2023
Aerolysin
General Aspects and function
Aerolysin is a toxin synthesized by some species of bacteria belonging to the genus Aeromonas, such as Aeromonas hydrophila. The exact function of Aerolysin may vary among different species and strains of Aeromonas. However, it is evident that it is the main macromolecule responsible for the pathogenicity of Aeromonas hydrophila, being associated with diarrheal diseases and deep wound infections [1]. About the structure of the protein, it is known that the toxin is setted up in an oligomeric structure with the activated forms of the monomers called proaerolysin.
Aerolysin plays several roles in the pathogenicity of Aeromonas sp. One of its main functions is its ability to promote lysis (rupture) of host cells, such as epithelial cells and immune cells. Aerolysin exhibits cytotoxic activity, causing damage to the cell membranes of host cells, which can lead to cell death and contribute to the bacterium's pathogenicity. Furthermore, aerolysin may be involved in the invasion and dissemination of the bacterium within the host. It can assist in tissue degradation, facilitating the bacterium's invasion into different organs and tissues of the host. Additionally, there are other proteins and virulence factors produced by Aeromonas spp. that also play important roles in the pathogenicity of these bacteria.
| |||||||||||
Biotechnological applications of aerolysin
In addition to its biological importance, aerolysin has also sparked interest in the field of nanotechnology due to its potential as a sensor in nanopore sequencing. In this technique, a nucleic acid molecule is passed through a nanopore, and individual nucleotide bases are detected and identified through characteristic changes in the electrical or ionic signal generated during the translocation. By observing the nature of aerolysin pores, some scientists have begun to explore the use of aerolysin pores in sequencing. Several aerolysin mutants used in nanopore sequencing have shown promising results, demonstrating excellent sensitivity, selectivity, and stability. [4]
References
- ↑ Altwegg M, Geiss HK. Aeromonas as a human pathogen. Crit Rev Microbiol. 1989;16(4):253-86. PMID:2649316 doi:10.3109/10408418909105478
- ↑ Parker MW, Buckley JT, Postma JP, Tucker AD, Leonard K, Pattus F, Tsernoglou D. Structure of the Aeromonas toxin proaerolysin in its water-soluble and membrane-channel states. Nature. 1994 Jan 20;367(6460):292-5. PMID:7510043 doi:http://dx.doi.org/10.1038/367292a0
- ↑ Iacovache I, De Carlo S, Cirauqui N, Dal Peraro M, van der Goot FG, Zuber B. Cryo-EM structure of aerolysin variants reveals a novel protein fold and the pore-formation process. Nat Commun. 2016 Jul 13;7:12062. doi: 10.1038/ncomms12062. PMID:27405240 doi:http://dx.doi.org/10.1038/ncomms12062
- ↑ Wang Y, Gu LQ, Tian K. The aerolysin nanopore: from peptidomic to genomic applications. Nanoscale. 2018 Aug 7;10(29):13857-13866. PMID:29998253 doi:10.1039/c8nr04255a