User:Marcos Vinícius Caetano/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 63: | Line 63: | ||
*'''Function''': Redirectionare the lever arm and contains a new CaM-binding motif. | *'''Function''': Redirectionare the lever arm and contains a new CaM-binding motif. | ||
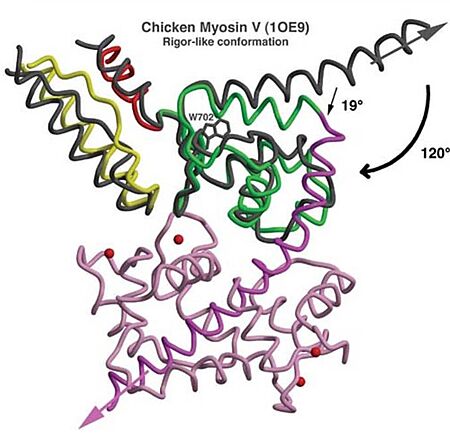
| - | *'''Mechanism''': The proximal part of insert 2 (<scene name='97/973101/774_812_distal_and_proximal/3'>Pro774-Trp787</scene> - in <span style="color:red">'''red'''</span>) wraps around the <scene name='97/973101/774_812_distal_and_proximal/4'>converter</scene> (in <span style="color:blue">'''blue'''</span>), while the distal part (<scene name='97/973101/774_812_distal_and_proximal/3'>Trp787-Tyr812</scene> - in <span style="color:green">'''green'''</span>) forms a CaM-binding motif. The insert 2 and its associated CaM molecule (with 4Ca<sup>2+</sup>), make specific interactions with the converter, many involving a variable loop (<scene name='97/973101/Insert_2_full/1'>Lys719-Pro731</scene>- in <span style="color:magenta">'''magenta'''</span>). The result of <scene name='97/973101/Insert_2_full/2'>interactions</scene> is that <scene name='97/973101/Iq_helix_emerge/1'>IQ helix</scene> - in <span style="color:green">'''green'''</span>) '''emerges ~120°''' from the position that it emerges in all other myosins, '''redirecting the IQ helix''' and the CaM towards the '''minus-end''' of the actin filament. The figure below compare the orientation of this IQ helix in myosin VI (green) with myosin V (black), and there is a difference of 19°, therefore, this is what makes myosin VI unique. | + | *'''Mechanism''': The proximal part of insert 2 (<scene name='97/973101/774_812_distal_and_proximal/3'>Pro774-Trp787</scene> - in <span style="color:red">'''red'''</span>) wraps around the <scene name='97/973101/774_812_distal_and_proximal/4'>converter</scene> (in <span style="color:blue">'''blue'''</span>), while the distal part (<scene name='97/973101/774_812_distal_and_proximal/3'>Trp787-Tyr812</scene> - in <span style="color:green">'''green'''</span>) forms a CaM-binding motif. The insert 2 and its associated CaM molecule (with 4Ca<sup>2+</sup>), make specific interactions with the converter, many involving a variable loop (<scene name='97/973101/Insert_2_full/1'>Lys719-Pro731</scene>- in <span style="color:magenta">'''magenta'''</span>). The result of <scene name='97/973101/Insert_2_full/2'>interactions</scene> is that <scene name='97/973101/Iq_helix_emerge/1'>IQ helix</scene> - in <span style="color:green">'''green'''</span>) '''emerges ~120°''' from the position that it emerges in all other myosins, '''redirecting the IQ helix''' and the CaM towards the '''minus-end''' of the actin filament. The figure below compare the orientation of this IQ helix in myosin VI (green) with myosin V (black), and there is a difference of 19°, therefore, '''this is what makes myosin VI unique'''. |
[[Image:Comparison MyoVI and MyoV.jpg|450px]] | [[Image:Comparison MyoVI and MyoV.jpg|450px]] | ||
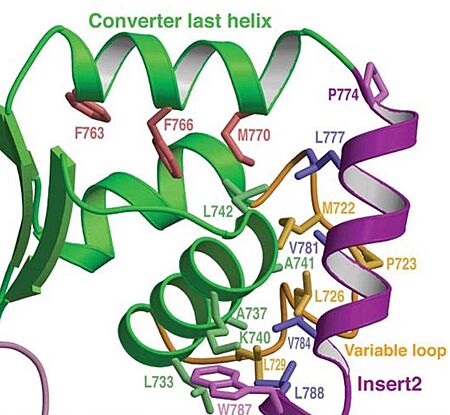
| - | In addiction, there is apolar interactions that stabilize the proximal part of insert 2 on the surface of the converter. Hidydrophofobic side chains from the amino acids (<span style="color:red">'''F63, F766, M770'''</span>)) stabilize the orientation of the last helix. | + | In addiction, there is '''apolar interactions''' that stabilize the proximal part of insert 2 on the surface of the converter. Hidydrophofobic side chains from the amino acids (<span style="color:red">'''F63, F766, M770'''</span>)) stabilize the orientation of the last helix. |
[[Image:Apolar interactions.jpg|450px]] | [[Image:Apolar interactions.jpg|450px]] | ||
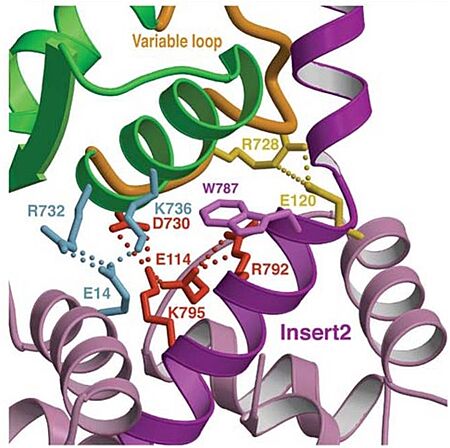
| - | Also, there is salt-bridge interactions between the converter and both lobes of CaM. | + | Also, there is '''salt-bridge interactions''' between the converter and both lobes of CaM. |
[[Image:Salt brigdes.jpg|450px]] | [[Image:Salt brigdes.jpg|450px]] | ||
Revision as of 08:47, 26 June 2023
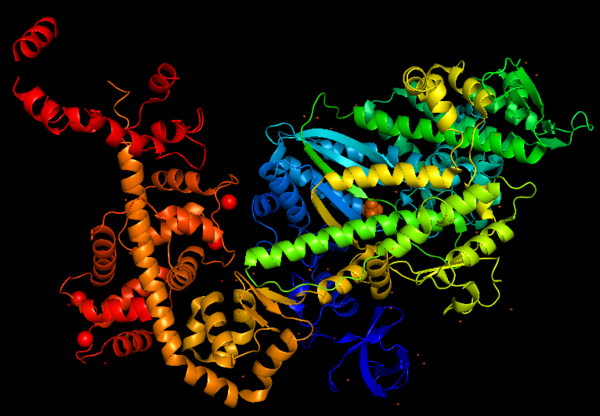
Myosin VI nucleotide-free (MDinsert2-IQ) crystal structure
| |||||||||||