We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Methane monooxygenase
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
== Function == | == Function == | ||
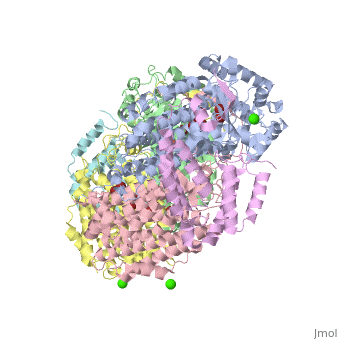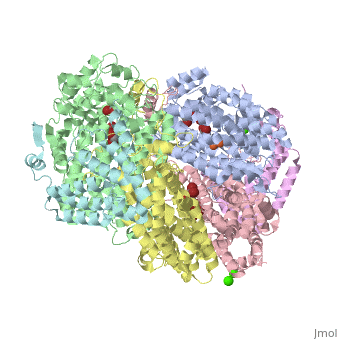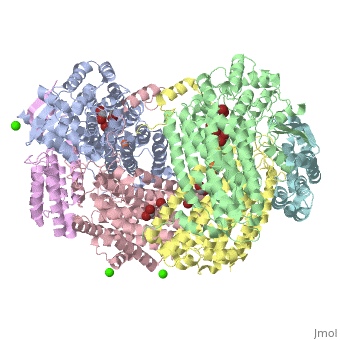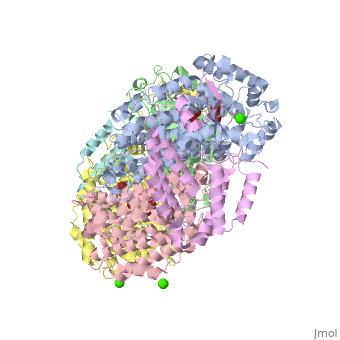
| - | '''Methane monooxygenase''' (MMO) catalyzes the oxidation of the C-H bond of methane and other alkanes. MMO catalyzes the conversion of CH<sub>4</sub> and O<sub>2</sub> to CH<sub>3</sub>OH and H<sub>2</sub>O using NADPH as reducing agent. The '''soluble MMO (sMMO)''' has Fe-O-Fe in its active site<ref>PMID:7826011</ref> while the '''particulate MMO (pMMO)''' has Cu in it<ref>PMID:21419924</ref>. MMO is composed of 3 subunits.<br /> | + | '''Methane monooxygenase''' or '''Methane monooxygenase hydroxyls''' (MMO) catalyzes the oxidation of the C-H bond of methane and other alkanes. MMO catalyzes the conversion of CH<sub>4</sub> and O<sub>2</sub> to CH<sub>3</sub>OH and H<sub>2</sub>O using NADPH as reducing agent. The '''soluble MMO (sMMO)''' has Fe-O-Fe in its active site<ref>PMID:7826011</ref> while the '''particulate MMO (pMMO)''' has Cu in it<ref>PMID:21419924</ref>. MMO is composed of 3 subunits.<br /> |
* Subunit α is the hydroxylase (MMOH) and contains the di-iron active site.<br /> | * Subunit α is the hydroxylase (MMOH) and contains the di-iron active site.<br /> | ||
* Subunit β is a reductase which contains the co-factors FAD and 2Fe-2S complex.<br /> | * Subunit β is a reductase which contains the co-factors FAD and 2Fe-2S complex.<br /> | ||
Revision as of 08:03, 28 June 2023
| |||||||||||
3D structures of methane monooxygenase
Updated on 28-June-2023
References
- ↑ Lipscomb JD. Biochemistry of the soluble methane monooxygenase. Annu Rev Microbiol. 1994;48:371-99. PMID:7826011 doi:http://dx.doi.org/10.1146/annurev.mi.48.100194.002103
- ↑ Miyaji A. Particulate methane monooxygenase from Methylosinus trichosporium OB3b. Methods Enzymol. 2011;495:211-25. doi: 10.1016/B978-0-12-386905-0.00014-0. PMID:21419924 doi:http://dx.doi.org/10.1016/B978-0-12-386905-0.00014-0