We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Lisinopril-Angiotensin Converting Enzyme
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | <StructureSection load= | + | <StructureSection load='' size='350' side='right' scene='74/745974/Lisinopril_stickandball/3' caption=''> |
'''Angiotensin Converting Enzyme''' is an enzyme that is found in the renin system that converts the hormone angiotensin I into angiotensin II which affects blood pressure by regulating the volume of fluids. The drug Lisinopril is an Angiotensin Converting Enzyme Inhibitor, which lowers the blood pressure in the body. | '''Angiotensin Converting Enzyme''' is an enzyme that is found in the renin system that converts the hormone angiotensin I into angiotensin II which affects blood pressure by regulating the volume of fluids. The drug Lisinopril is an Angiotensin Converting Enzyme Inhibitor, which lowers the blood pressure in the body. | ||
| Line 13: | Line 13: | ||
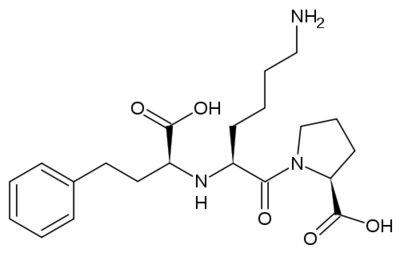
<scene name='74/745974/Lisinopril_2/1'>Lisinopril</scene> <ref>Canner, D. Lisinopril http://proteopedia.org/wiki/index.php/prinivil (accessed May 2, 2019).</ref> (S)-1-[(N^2-carboxyl-3-phenylpropyl]-L-lysyl-L-proline diyhdrate) is a drug was patented by Merck and Co in 1987. It is a drug that is used to inhibit the Angiotensin converting enzyme to treat hypertension, congestive heart failure and improve survival after a heart attack. Its molecular formula is C21H31N3O5•2H2O. This drug is soluble in water, and is insoluble in ethanol and slightly soluble in methanol.This is a prescription only medication that comes in three different strengths, 5, 10, 20mg. The active ingredient in this drug is Lisinopril but there are a few inactive ingredients. Those of which include, calcium phosphate, mannitol, magnesium stearate and starch. These inactive ingredients are in all three strengths however, in the 10mg and 20mg strength there is an extra ingredient added which is iron oxide. <ref>PRINIVIL® (lisinopril) https://www.merck.com/product/usa/pi_circulars/p/prinivil/prinivil_pi.pdf</ref> | <scene name='74/745974/Lisinopril_2/1'>Lisinopril</scene> <ref>Canner, D. Lisinopril http://proteopedia.org/wiki/index.php/prinivil (accessed May 2, 2019).</ref> (S)-1-[(N^2-carboxyl-3-phenylpropyl]-L-lysyl-L-proline diyhdrate) is a drug was patented by Merck and Co in 1987. It is a drug that is used to inhibit the Angiotensin converting enzyme to treat hypertension, congestive heart failure and improve survival after a heart attack. Its molecular formula is C21H31N3O5•2H2O. This drug is soluble in water, and is insoluble in ethanol and slightly soluble in methanol.This is a prescription only medication that comes in three different strengths, 5, 10, 20mg. The active ingredient in this drug is Lisinopril but there are a few inactive ingredients. Those of which include, calcium phosphate, mannitol, magnesium stearate and starch. These inactive ingredients are in all three strengths however, in the 10mg and 20mg strength there is an extra ingredient added which is iron oxide. <ref>PRINIVIL® (lisinopril) https://www.merck.com/product/usa/pi_circulars/p/prinivil/prinivil_pi.pdf</ref> | ||
| - | [[Image:Lisinopril.png]] | + | [[Image:Lisinopril.png|left|thumb|400px]] |
| + | {{Clear}} | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
| Line 21: | Line 22: | ||
ACE inhibitors are used for many different treatments of cardiovascular and renal diseases. These drugs are used to alter the balance of a few different systems including, vasoconstrictive, salt- retentive and hyperopic properties. Lisinopril is one of the drugs that is used to treat hypertension however it is not just effecting the blood pressure. When Lisinopril is introduced into the body and attaches to the active site of the angiotensin converting enzyme through competitive inhibition it causes the levels of bradykinin found with kinase which is very similar to ACE, in the blood stream to rise. This is because ACE helps break down bradykinin and when Lisinopril is used the enzyme is inhibited which is also inhibiting the breakdown of bradykinin. So not only does Lisinopril cause a decrease in blood pressure but it also causes an increase in vasodilation.<ref>Brown , Nancy J, and Douglas E Vaughan. “Angiotensin-Converting Enzyme Inhibitors.” Circulation, www.ahajournals.org/doi/10.1161/01.CIR.97.14.1411. | ACE inhibitors are used for many different treatments of cardiovascular and renal diseases. These drugs are used to alter the balance of a few different systems including, vasoconstrictive, salt- retentive and hyperopic properties. Lisinopril is one of the drugs that is used to treat hypertension however it is not just effecting the blood pressure. When Lisinopril is introduced into the body and attaches to the active site of the angiotensin converting enzyme through competitive inhibition it causes the levels of bradykinin found with kinase which is very similar to ACE, in the blood stream to rise. This is because ACE helps break down bradykinin and when Lisinopril is used the enzyme is inhibited which is also inhibiting the breakdown of bradykinin. So not only does Lisinopril cause a decrease in blood pressure but it also causes an increase in vasodilation.<ref>Brown , Nancy J, and Douglas E Vaughan. “Angiotensin-Converting Enzyme Inhibitors.” Circulation, www.ahajournals.org/doi/10.1161/01.CIR.97.14.1411. | ||
</ref>This often causes the person taking the medication of have a dry cough as a side effect of the medication. | </ref>This often causes the person taking the medication of have a dry cough as a side effect of the medication. | ||
| - | + | </StructureSection> | |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 12:21, 7 August 2023
| |||||||||||
References
- ↑ Akif M, Georgiadis D, Mahajan A, Dive V, Sturrock ED, Isaac RE, Acharya KR. High-resolution crystal structures of Drosophila melanogaster angiotensin-converting enzyme in complex with novel inhibitors and antihypertensive drugs. J Mol Biol. 2010 Jul 16;400(3):502-17. Epub 2010 May 19. PMID:20488190 doi:10.1016/j.jmb.2010.05.024
- ↑ Riordan, James F. “Angiotensin-I-Converting Enzyme and Its Relatives.” Genome Biology, BioMed Central, 2003, www.ncbi.nlm.nih.gov/pmc/articles/PMC193637/.
- ↑ Sturrock, E.D., Natesh, R., van Rooyen, J.M. et al. CMLS, Cell. Mol. Life Sci. (2004) 61: 2677. https://doi.org/10.1007/s00018-004-4239-0
- ↑ Canner, D. Lisinopril http://proteopedia.org/wiki/index.php/prinivil (accessed May 2, 2019).
- ↑ PRINIVIL® (lisinopril) https://www.merck.com/product/usa/pi_circulars/p/prinivil/prinivil_pi.pdf
- ↑ Lopez, Edgardo Olvera. “Lisinopril.” StatPearls [Internet]., U.S. National Library of Medicine, 20 Jan. 2019, www.ncbi.nlm.nih.gov/books/NBK482230/
- ↑ Sondes Bouabdallah, Med Thaieb Ben Dhia, and Med Rida Driss, “Study of a Conformational Equilibrium of Lisinopril by HPLC, NMR, and DFT,” International Journal of Analytical Chemistry, vol. 2014, Article ID 494719, 8 pages, 2014. https://doi.org/10.1155/2014/494719.
- ↑ http://proteopedia.org/wiki/index.php/ACE_Inhibitor_Prinivil
- ↑ Sturrock, E D, et al. “Structure of Angiotensin I-Converting Enzyme.” Cellular and Molecular Life Sciences, vol. 61, 2004, doi:10.18411/a-2017-023.
- ↑ Brown , Nancy J, and Douglas E Vaughan. “Angiotensin-Converting Enzyme Inhibitors.” Circulation, www.ahajournals.org/doi/10.1161/01.CIR.97.14.1411.