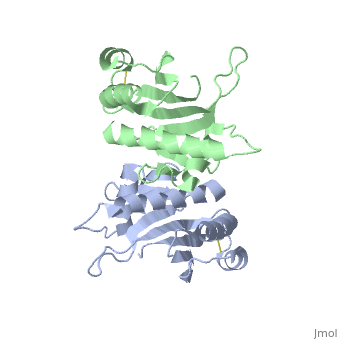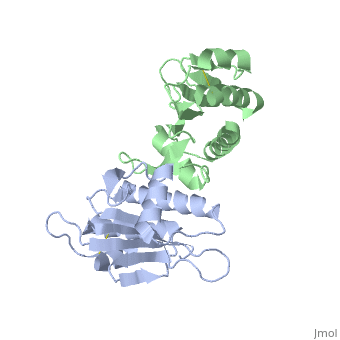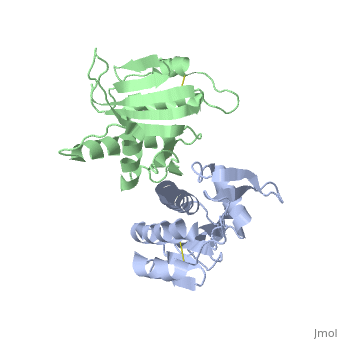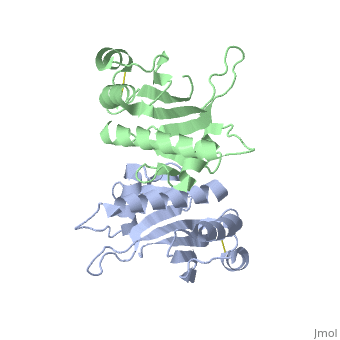We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Pilin
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
==Structure== | ==Structure== | ||
| - | Type IV pilin are homopolymers, composed of a single-chain pilin protein. The first common element that all type IVb pilins contain is the inclusion of the C-terminal β-strand (β7 in PilS) in the center of the structure, forming an antiparallel arrangement. <ref name=journal1>PMID:19626704</ref> A conserved 25 residue hydrophobic N terminal sequence, that serves as an oligomerization domain for fibre formation, is shared by all type IV pilins. Finally, all pilins also share a conserved pilus assembly machinery, a unique N-methylated N-terminus and a pair of conserved cysteines that form an intrachain disulphide bond. The type IVb pilus operon of ''S. typhi'' contains the piLS gene, which encodes a structural pilin. <ref name=journal1>PMID:19626704</ref> | + | Type IV pilin are homopolymers, composed of a single-chain pilin protein called '''major pilin''' and a less abundant protein called '''minor pilin''' which are essential for the pilus assembly and function<ref name=journal1>PMID:31784891</ref>. The first common element that all type IVb pilins contain is the inclusion of the C-terminal β-strand (β7 in PilS) in the center of the structure, forming an antiparallel arrangement. <ref name=journal1>PMID:19626704</ref> A conserved 25 residue hydrophobic N terminal sequence, that serves as an oligomerization domain for fibre formation, is shared by all type IV pilins. Finally, all pilins also share a conserved pilus assembly machinery, a unique N-methylated N-terminus and a pair of conserved cysteines that form an intrachain disulphide bond. The type IVb pilus operon of ''S. typhi'' contains the piLS gene, which encodes a structural pilin. <ref name=journal1>PMID:19626704</ref> |
The cysteine-containing region in the C-terminus is thought to play a function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> The D-region of the type IVb pilins is known to contain residues that function in pilus assembly; this region is stabilized by a conserved disulfide bond. <ref name=journal1>PMID:19626704</ref> | The cysteine-containing region in the C-terminus is thought to play a function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> The D-region of the type IVb pilins is known to contain residues that function in pilus assembly; this region is stabilized by a conserved disulfide bond. <ref name=journal1>PMID:19626704</ref> | ||
Revision as of 11:01, 30 August 2023
| |||||||||||
Additional Resources
To See additional information, see: Bacterial Infections
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Balakrishna AM, Saxena AM, Mok HY, Swaminathan K. Structural basis of typhoid: Salmonella typhi type IVb pilin (PilS) and cystic fibrosis transmembrane conductance regulator interaction. Proteins. 2009 Nov 1;77(2):253-61. PMID:19626704 doi:10.1002/prot.22500
- ↑ 2.0 2.1 2.2 Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Elyse Yaremco, David Canner, Andrea Gorrell, Alexander Berchansky