Drastic alterations in the loop structure around colchicine upon complex formation with an engineered lipocalin indicate a conformational selection mechanism
Elena Jerschke, Andreas Eichinger and Arne Skerra [1]
Molecular Tour
Natural lipocalin proteins are found in diverse phyla of life and are involved in the transport and storage of vitamins, lipids or microbial siderophores. Despite very low mutual amino acid sequence identity, the members of this protein family exhibit a structurally highly conserved fold which consists of a beta-barrel with an adjacent alpha-helix and a cup-shaped binding pocket which is formed by four structurally variable loops at the open end. Reshaping this binding pocket by combinatorial protein design leads to so-called Anticalins, a novel class of tailor-made lipocalins with novel binding functions that offer prospects for medical application. Previous structural analyses have revealed evidence that the binding mode of engineered as well as natural lipocalins to their target molecules resembles the interaction between antibodies (also known as immunoglobulins) and their antigens. Apart from a simple rigid lock-and-key binding interaction, antibodies are known for two mechanisms of spatial adaptation when forming complexes with antigens: (i) induced fit, in which conformational alteration follows ligand binding, and (ii) conformational selection, which is based on a pre-existing mixture of conformational states.
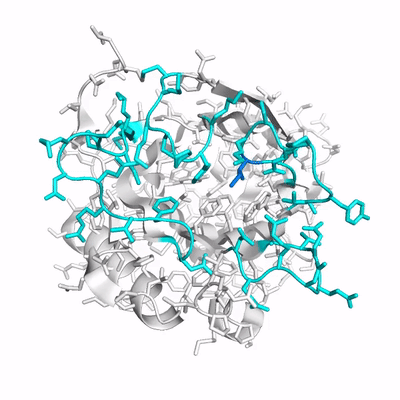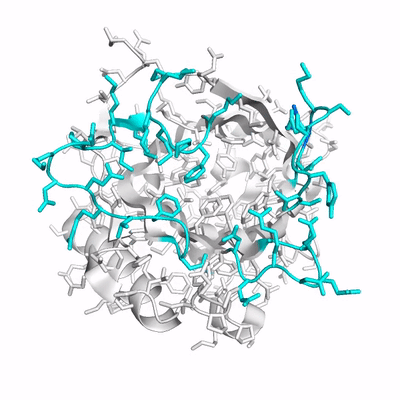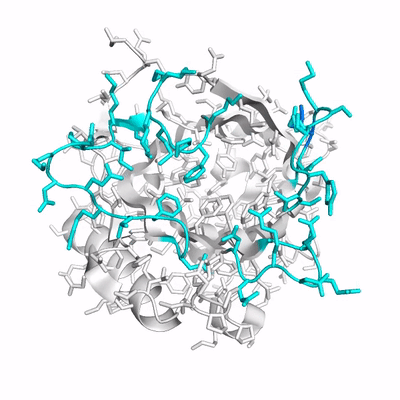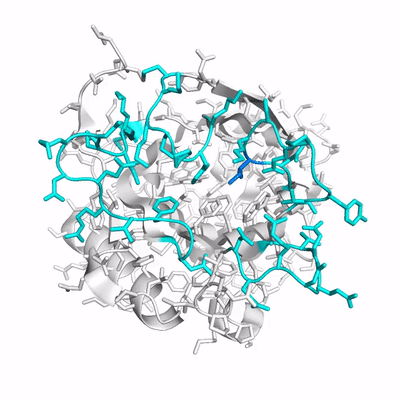
Up to now, studies of Anticalins have mainly provided examples for an induced fit mechanism. Here, we have investigated the crystal structure of Colchicalin, an Anticalin with picomolar affinity and neutralizing activity towards the plant poison colchicine, in its ligand-free state (PDB ID: 6z6z) and compared this with the previously solved structure of its colchicine complex (PDB ID: 5nkn):
- (PDB ID: 5nkn; colchicine colored in orange and loops colored magenta).
- (PDB ID: 6z6z; loops colored cyan).
Surprisingly, a revealed a largely occluded binding pocket in the unliganded protein.
As result of a in particular of the at its tip occupies part of the binding site and, thus, would interfere with ligand binding. Conversely, in order to liberate the necessary space for colchicine, a dramatic shift of loop #3 by 11 Å, in combination with a side chain flip of Phe71 on the neighboring loop #2 is required. Consequently, one has to assume that the open conformation of Colchicalin must already exist, even though at a low proportion, in the absence of the ligand to enable its binding, which is then followed by a shift in the equilibrium between the closed and open protein states. Hence, the mechanism of conformational selection appears to adequately describe the mode of ligand binding for this Anticalin. . Prior to complex formation, Colchicalin exists in solution in at least two states, one with an open and one with an occluded ligand pocket, and only the open state can accommodate colchicine. Accordingly, the Colchicalin-colchicine pair provides another example for the analogy between (engineered) lipocalins and antibodies with regard to the mechanism of ligand/antigen recognition.
References
- ↑ doi: https://dx.doi.org/10.1107/S2053230X23006817

