|
|
| Line 3: |
Line 3: |
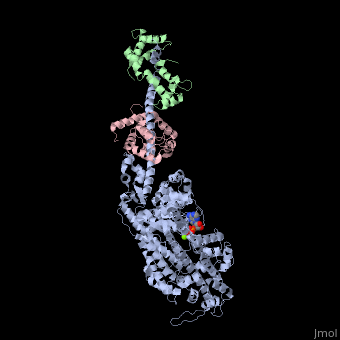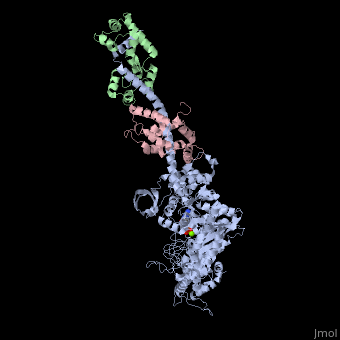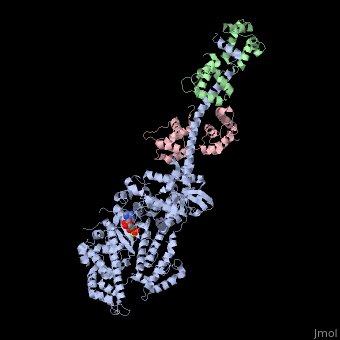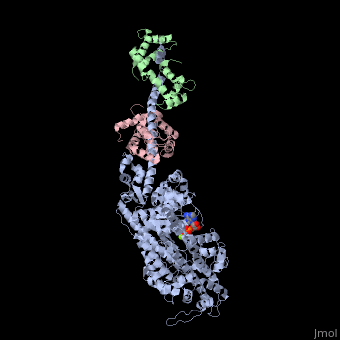
| | <StructureSection load='3i5f' size='340' side='right'caption='[[3i5f]], [[Resolution|resolution]] 3.10Å' scene=''> | | <StructureSection load='3i5f' size='340' side='right'caption='[[3i5f]], [[Resolution|resolution]] 3.10Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[3i5f]] is a 3 chain structure with sequence from [http://en.wikipedia.org/wiki/Loligo_pealei Loligo pealei] and [http://en.wikipedia.org/wiki/Todarodes_pacificus Todarodes pacificus]. This structure supersedes the now removed PDB entry [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=2oy6 2oy6]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3I5F OCA]. For a <b>guided tour on the structure components</b> use [http://proteopedia.org/fgij/fg.htm?mol=3I5F FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[3i5f]] is a 3 chain structure with sequence from [https://en.wikipedia.org/wiki/Doryteuthis_pealeii Doryteuthis pealeii] and [https://en.wikipedia.org/wiki/Todarodes_pacificus Todarodes pacificus]. This structure supersedes the now removed PDB entry [http://oca.weizmann.ac.il/oca-bin/send-pdb?obs=1&id=2oy6 2oy6]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3I5F OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3I5F FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ADP:ADENOSINE-5-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.1Å</td></tr> |
| - | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2oy6|2oy6]], [[3i5g|3i5g]], [[3i5h|3i5h]], [[3i5i|3i5i]]</td></tr> | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=ADP:ADENOSINE-5-DIPHOSPHATE'>ADP</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://proteopedia.org/fgij/fg.htm?mol=3i5f FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3i5f OCA], [http://pdbe.org/3i5f PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=3i5f RCSB], [http://www.ebi.ac.uk/pdbsum/3i5f PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=3i5f ProSAT]</span></td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3i5f FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3i5f OCA], [https://pdbe.org/3i5f PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3i5f RCSB], [https://www.ebi.ac.uk/pdbsum/3i5f PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3i5f ProSAT]</span></td></tr> |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/MLE_TODPA MLE_TODPA]] In molluscan muscle, calcium regulation is associated with myosin rather than with actin. Muscle myosin contains two types of light chains: the catalytic light chain, essential for ATPase activity, and the regulatory light chain, a calcium-binding protein responsible for Ca(2+) dependent binding and Ca(2+) dependent Mg-ATPase activity. [[http://www.uniprot.org/uniprot/MLR_TODPA MLR_TODPA]] In molluscan muscle, calcium regulation is associated with myosin rather than with actin. Muscle myosin contains two types of light chains: the catalytic light chain, essential for ATPase activity, and the regulatory light chain, a calcium-binding protein responsible for Ca(2+) dependent binding and Ca(2+) dependent Mg-ATPase activity. | + | [https://www.uniprot.org/uniprot/O44934_DORPE O44934_DORPE] |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 37: |
Line 37: |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| | + | [[Category: Doryteuthis pealeii]] |
| | [[Category: Large Structures]] | | [[Category: Large Structures]] |
| - | [[Category: Loligo pealei]] | |
| | [[Category: Todarodes pacificus]] | | [[Category: Todarodes pacificus]] |
| - | [[Category: Brown, J H]] | + | [[Category: Brown JH]] |
| - | [[Category: Cohen, C]] | + | [[Category: Cohen C]] |
| - | [[Category: Gourinath, S]] | + | [[Category: Gourinath S]] |
| - | [[Category: Himmel, D M]] | + | [[Category: Himmel DM]] |
| - | [[Category: Kovacs, M]] | + | [[Category: Kovacs M]] |
| - | [[Category: Neall-Hennessey, E O]] | + | [[Category: Nyitray L]] |
| - | [[Category: Nyitray, L]] | + | [[Category: O'Neall-Hennessey E]] |
| - | [[Category: Reshetnikova, L]] | + | [[Category: Reshetnikova L]] |
| - | [[Category: Reutzel, R]] | + | [[Category: Reutzel R]] |
| - | [[Category: Szent-Gyorgyi, A G]] | + | [[Category: Szent-Gyorgyi AG]] |
| - | [[Category: Yang, Y]] | + | [[Category: Yang Y]] |
| - | [[Category: Actin-binding]]
| + | |
| - | [[Category: Atp-binding]]
| + | |
| - | [[Category: Calcium]]
| + | |
| - | [[Category: Coiled coil]]
| + | |
| - | [[Category: Contractile protein]]
| + | |
| - | [[Category: Mg adp]]
| + | |
| - | [[Category: Motor protein]]
| + | |
| - | [[Category: Muscle protein]]
| + | |
| - | [[Category: Myosin]]
| + | |
| - | [[Category: Myosin ii s1]]
| + | |
| - | [[Category: Nucleotide-binding]]
| + | |
| - | [[Category: Post-rigor state]]
| + | |
| - | [[Category: Squid]]
| + | |
| - | [[Category: Thick filament]]
| + | |
| Structural highlights
Function
O44934_DORPE
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Unlike processive cellular motors such as myosin V, whose structure has recently been determined in a "rigor-like" conformation, myosin II from contracting muscle filaments necessarily spends most of its time detached from actin. By using squid and sea scallop sources, however, we have now obtained similar rigor-like atomic structures for muscle myosin heads (S1). The significance of the hallmark closed actin-binding cleft in these crystal structures is supported here by actin/S1-binding studies. These structures reveal how different duty ratios, and hence cellular functions, of the myosin isoforms may be accounted for, in part, on the basis of detailed differences in interdomain contacts. Moreover, the rigor-like position of switch II turns out to be unique for myosin V. The overall arrangements of subdomains in the motor are relatively conserved in each of the known contractile states, and we explore qualitatively the energetics of these states.
Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor.,Yang Y, Gourinath S, Kovacs M, Nyitray L, Reutzel R, Himmel DM, O'Neall-Hennessey E, Reshetnikova L, Szent-Gyorgyi AG, Brown JH, Cohen C Structure. 2007 May;15(5):553-64. PMID:17502101[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Yang Y, Gourinath S, Kovacs M, Nyitray L, Reutzel R, Himmel DM, O'Neall-Hennessey E, Reshetnikova L, Szent-Gyorgyi AG, Brown JH, Cohen C. Rigor-like structures from muscle myosins reveal key mechanical elements in the transduction pathways of this allosteric motor. Structure. 2007 May;15(5):553-64. PMID:17502101 doi:10.1016/j.str.2007.03.010
|