We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
5jk7
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
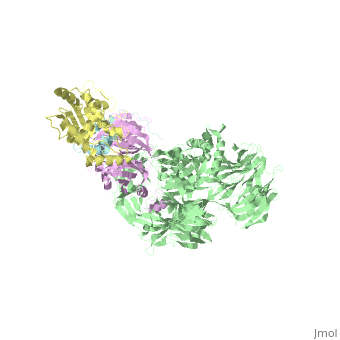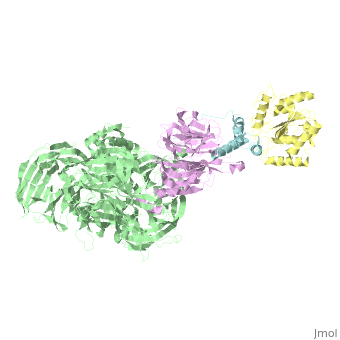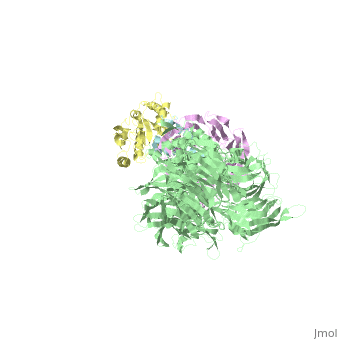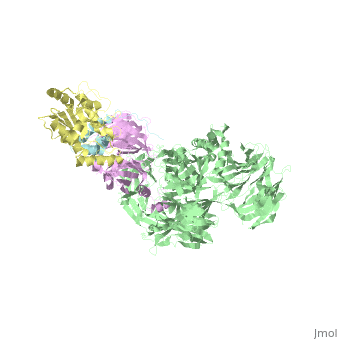
<StructureSection load='5jk7' size='340' side='right'caption='[[5jk7]], [[Resolution|resolution]] 3.49Å' scene=''> | <StructureSection load='5jk7' size='340' side='right'caption='[[5jk7]], [[Resolution|resolution]] 3.49Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[5jk7]] is a 8 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[5jk7]] is a 8 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens] and [https://en.wikipedia.org/wiki/Human_immunodeficiency_virus_type_1_(NEW_YORK-5_ISOLATE) Human immunodeficiency virus type 1 (NEW YORK-5 ISOLATE)]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=5JK7 OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=5JK7 FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.49Å</td></tr> |
| - | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=5jk7 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=5jk7 OCA], [https://pdbe.org/5jk7 PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=5jk7 RCSB], [https://www.ebi.ac.uk/pdbsum/5jk7 PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=5jk7 ProSAT]</span></td></tr> | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | |
</table> | </table> | ||
| - | == Disease == | ||
| - | [[http://www.uniprot.org/uniprot/UNG_HUMAN UNG_HUMAN]] Defects in UNG are a cause of immunodeficiency with hyper-IgM type 5 (HIGM5) [MIM:[http://omim.org/entry/608106 608106]]. A rare immunodeficiency syndrome characterized by normal or elevated serum IgM levels with absence of IgG, IgA, and IgE. It results in a profound susceptibility to bacterial infections.<ref>PMID:12958596</ref> <ref>PMID:15967827</ref> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/DDB1_HUMAN DDB1_HUMAN] Required for DNA repair. Binds to DDB2 to form the UV-damaged DNA-binding protein complex (the UV-DDB complex). The UV-DDB complex may recognize UV-induced DNA damage and recruit proteins of the nucleotide excision repair pathway (the NER pathway) to initiate DNA repair. The UV-DDB complex preferentially binds to cyclobutane pyrimidine dimers (CPD), 6-4 photoproducts (6-4 PP), apurinic sites and short mismatches. Also appears to function as a component of numerous distinct DCX (DDB1-CUL4-X-box) E3 ubiquitin-protein ligase complexes which mediate the ubiquitination and subsequent proteasomal degradation of target proteins. The functional specificity of the DCX E3 ubiquitin-protein ligase complex is determined by the variable substrate recognition component recruited by DDB1. DCX(DDB2) (also known as DDB1-CUL4-ROC1, CUL4-DDB-ROC1 and CUL4-DDB-RBX1) may ubiquitinate histone H2A, histone H3 and histone H4 at sites of UV-induced DNA damage. The ubiquitination of histones may facilitate their removal from the nucleosome and promote subsequent DNA repair. DCX(DDB2) also ubiquitinates XPC, which may enhance DNA-binding by XPC and promote NER. DCX(DTL) plays a role in PCNA-dependent polyubiquitination of CDT1 and MDM2-dependent ubiquitination of TP53 in response to radiation-induced DNA damage and during DNA replication. DCX(ERCC8) (the CSA complex) plays a role in transcription-coupled repair (TCR). May also play a role in ubiquitination of CDKN1B/p27kip when associated with CUL4 and SKP2.<ref>PMID:12732143</ref> <ref>PMID:15448697</ref> <ref>PMID:14739464</ref> <ref>PMID:15882621</ref> <ref>PMID:16260596</ref> <ref>PMID:16482215</ref> <ref>PMID:17079684</ref> <ref>PMID:16407242</ref> <ref>PMID:16407252</ref> <ref>PMID:16678110</ref> <ref>PMID:16940174</ref> <ref>PMID:17041588</ref> <ref>PMID:16473935</ref> <ref>PMID:18593899</ref> <ref>PMID:18381890</ref> <ref>PMID:18332868</ref> |
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
== Publication Abstract from PubMed == | == Publication Abstract from PubMed == | ||
| Line 32: | Line 29: | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Homo sapiens]] |
| - | + | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | + | [[Category: Ahn J]] | |
| - | [[Category: Ahn | + | [[Category: Calero G]] |
| - | [[Category: Calero | + | [[Category: Wu Y]] |
| - | [[Category: Wu | + | |
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 18:56, 20 September 2023
The X-ray structure of the DDB1-DCAF1-Vpr-UNG2 complex
| |||||||||||
Categories: Homo sapiens | Large Structures | Ahn J | Calero G | Wu Y